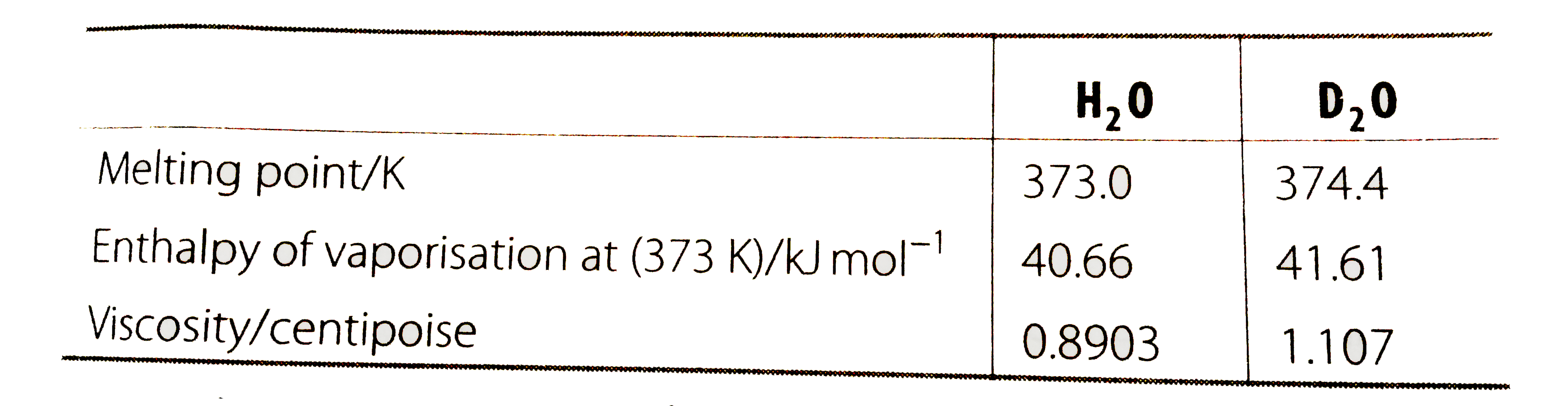Similar Questions
Explore conceptually related problems
Recommended Questions
- Melting point, enthaply of vaporisation and visvocsity data of H(2...
Text Solution
|
- For the given cell Pt(D(2)|D^(o+))||H^(o+)|Pt(H(2)) , if E^(c-).(D(2)|...
Text Solution
|
- Which is true statement about D(2)O and H(2)O ?
Text Solution
|
- Assertion :D(2)O has higher boiling point than H(2)O. Reason : Visco...
Text Solution
|
- Which of the following statements are correct regarding D(2)O and H...
Text Solution
|
- Melting point, enthaply of vaporisation and visvocsity data of H(2)O a...
Text Solution
|
- H(2)S is a stronger acid than H(2)O . Explain
Text Solution
|
- Melting point, enthalpy of vapourisation and viscosity data of H(2)O a...
Text Solution
|
- H(2)O एवं D(2)O के गुणों को जानते हुए क्या आप मानते है कि D(2)O का उपय...
Text Solution
|