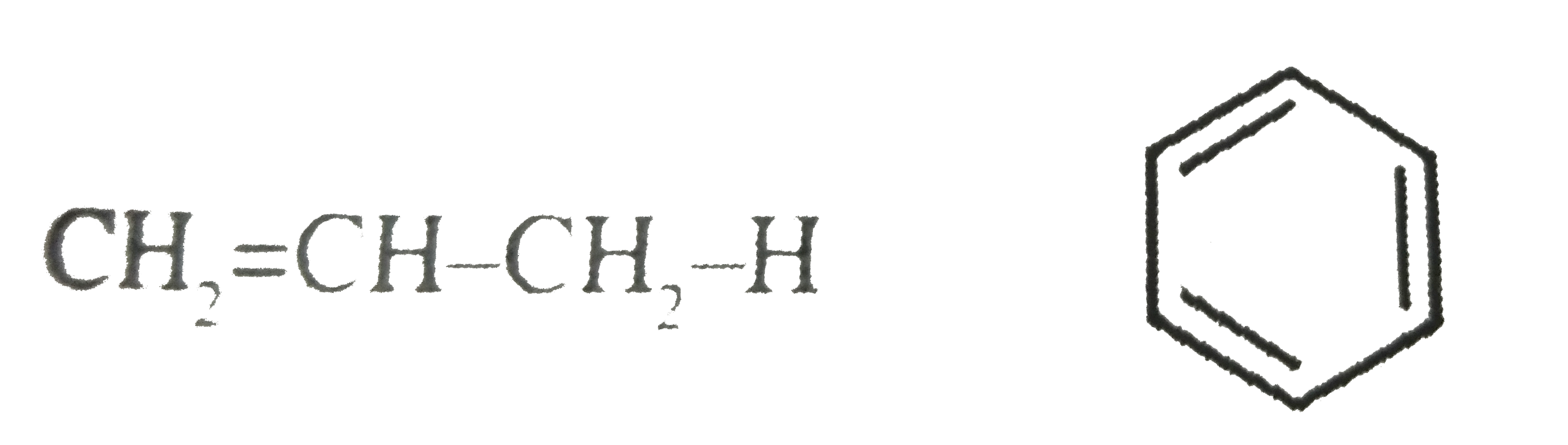Similar Questions
Explore conceptually related problems
Recommended Questions
- The decreasing order of bond dissociation energy of C-H bond in given ...
Text Solution
|
- In which of the following compounds corban atom undergoes hybridisati...
Text Solution
|
- CH(3)-.^(2)underset(.(1)CH(2)-H)underset(|)overset(H)overset(|)C-.^(3)...
Text Solution
|
- The bond dissociation energies of the following underset(I)(CH(3)-H) u...
Text Solution
|
- Which is the most suitable reagent for the following transformation ? ...
Text Solution
|
- The decreasing order of bond dissociation energy of C-H bond in given ...
Text Solution
|
- In a series ethane (CH(3)-CH(3)) ethylene (CH(2)=CH(2)) and acetylene ...
Text Solution
|
- Correct name for the given compound CH(3)-CH(2)-underset(CH(2)CH(3))...
Text Solution
|
- Arrange the following compounds in order of increasing bond dissociati...
Text Solution
|