Similar Questions
Explore conceptually related problems
Recommended Questions
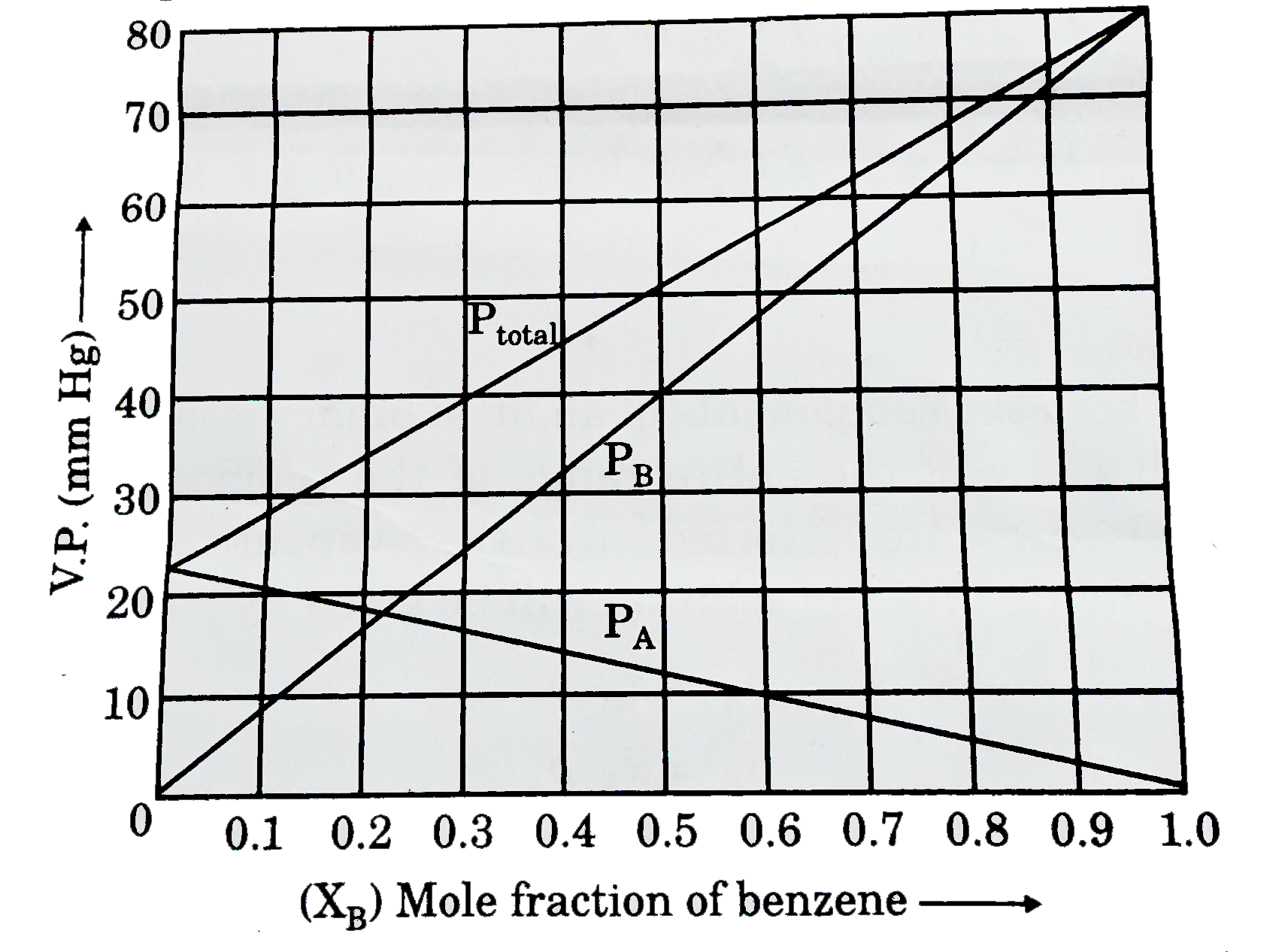
- Answer the questions (given below) which are based on the following di...
Text Solution
|
- Benzene and toluene form nearly ideal solution. At 298 K, the vapour p...
Text Solution
|
- A mixture of benzene and toluence forms
Text Solution
|
- Benzene and toluene form nearly ideal solutions. At 20^(@) C, the vapo...
Text Solution
|
- Answer the questions (given below) which are based on the following di...
Text Solution
|
- Answer the questions (given below) which are based on the following di...
Text Solution
|
- Consider the following partially labelled figure (graph is upto scale)...
Text Solution
|
- Consider the following partially labelled figure (graph is upto scale)...
Text Solution
|
- Consider the following partially labelled figure (graph is upto scale)...
Text Solution
|