Similar Questions
Explore conceptually related problems
Recommended Questions
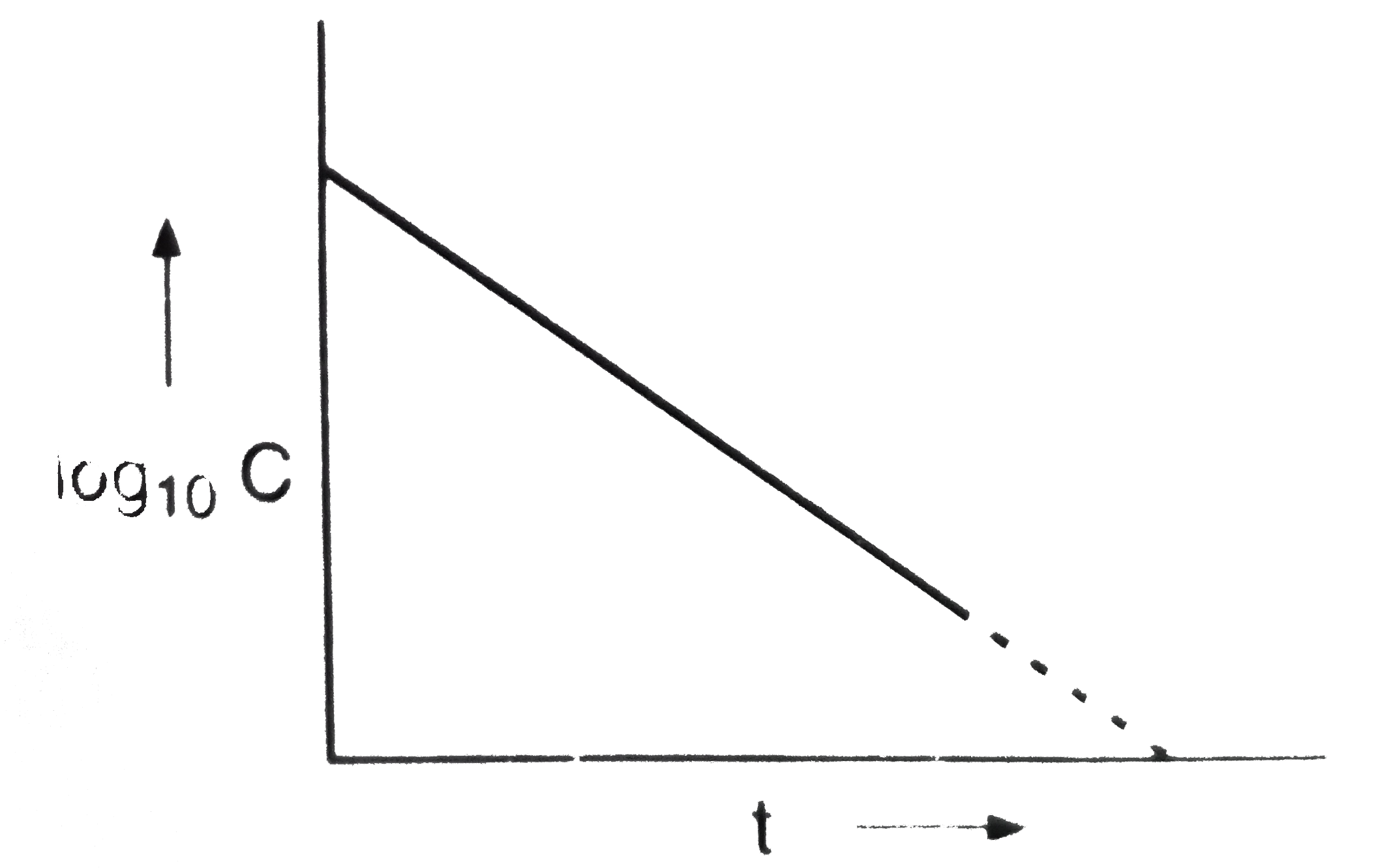
- If a plant of log(10) C versust t gives a straight line for a given re...
Text Solution
|
- For a certain reaction, a plot of ([C(0)-C])/(C ) against time t , yie...
Text Solution
|
- For a first order reaction, the reaction, the plot of log C against 't...
Text Solution
|
- Which straight line gives the activation energy for a reaction?
Text Solution
|
- If the graph of concentration of [A] vs T for completion of reaction ...
Text Solution
|
- For first order gaseous reaction , log k when plotted atgains (1)/(t) ...
Text Solution
|
- If a plant of log(10) C versust t gives a straight line for a given re...
Text Solution
|
- In the reaction A+BrarrC+D, the rate ((dx)/(dt)) when plotted against ...
Text Solution
|
- यदि log(10)[A] तथा समय के मध्य आरेखित ग्राफ तृणात्मक प्रवणता के साथ सी...
Text Solution
|