Similar Questions
Explore conceptually related problems
Recommended Questions
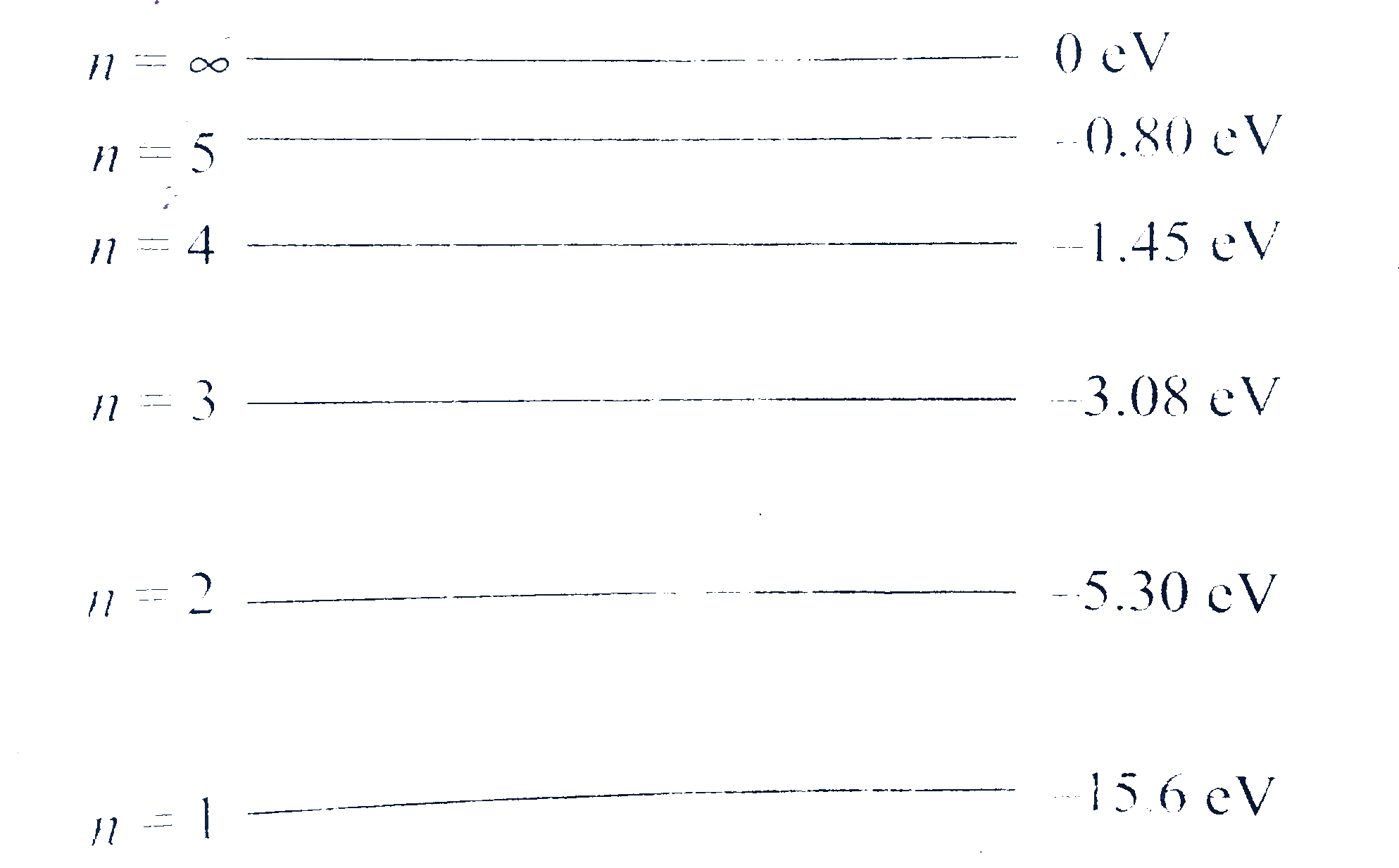
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- The energy levels of a hypotherical one electron atom are shown in fig...
Text Solution
|
- 16 eV ऊर्जा का फोटॉन हाइड्रोजन परमाणु को मूल-ऊर्जा स्तर में आयनित करता...
Text Solution
|
- हाइड्रोजन परमाणु की मूल ऊर्जा स्तर की ऊर्जा - 13*6 eV हैं इस अवस्था मे...
Text Solution
|
- An one -electron atom has an energy of -3.4 eV. The kinetic energy of...
Text Solution
|