Similar Questions
Explore conceptually related problems
Recommended Questions
- Henry’s constant (in kbar) for four gases alpha, beta, gamma and delta...
Text Solution
|
- If the degree of ionization of water be 1.8xx10^(-9) at 298 K. Its ion...
Text Solution
|
- The concentration of water molecules in pure water at 298 K is
Text Solution
|
- 298 K ताप पर जल का pH होगा
Text Solution
|
- For the solution of the gases W, X, Y and z in water at 298 K , the He...
Text Solution
|
- The degree of dissociation of water is 1.8 xx10^(-9) at 298 K . Calcul...
Text Solution
|
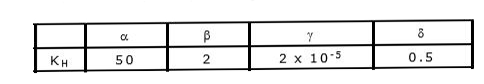
- Four gases alpha, beta, gamma and delta have KH values 50kbar, 20 kbar...
Text Solution
|
- The value of Henry's law constant for few gases at 298 K is given belo...
Text Solution
|
- Henry’s constant (in kbar) for four gases alpha, beta, gamma and delta...
Text Solution
|