Similar Questions
Explore conceptually related problems
Recommended Questions
- For an atom of an ion having single electron, the wavelengths observed...
Text Solution
|
- If lambda(1) and lambda(2) denote the wavelength of de-broglie waves f...
Text Solution
|
- For an atom of an ion having single electron, the wavelengths observed...
Text Solution
|
- If lambda(1), lambda(2), lambda(3) are the wavelengths of the waves gi...
Text Solution
|
- Let be the maximum kinetic energy of photoelectrons emitted by light o...
Text Solution
|
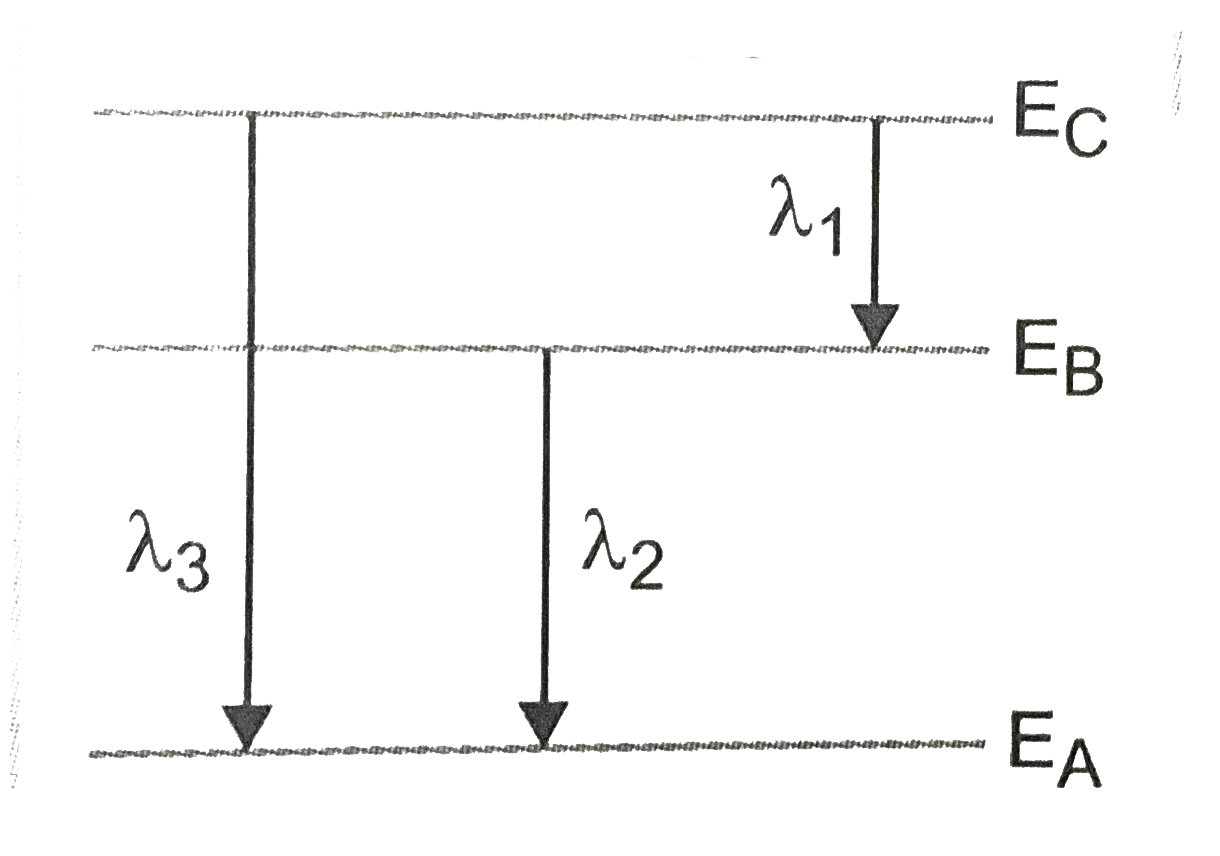
- In hydrogen atom, if lambda(1),lambda(2),lambda(3) are shortest wavel...
Text Solution
|
- The wavelength of electron waves in two orbits (lambda(1) : lambda(2))
Text Solution
|
- For balmer series wavelength of first line is lambda(1) and for bracke...
Text Solution
|
- If the matrix A = [[lambda(1)^(2), lambda(1)lambda(2), lambda(1) lambd...
Text Solution
|