Similar Questions
Explore conceptually related problems
Recommended Questions
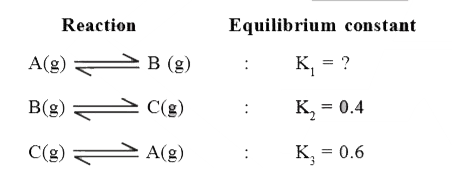
- The equilibrium between, gaseous isomers A, B and C can be represented...
Text Solution
|
- 1 mole hydrogen and 2 moles oxygen are filled in neigboring parts of a...
Text Solution
|
- The equilibrium between, gaseous isomers A,B and C can be represented ...
Text Solution
|
- One mole of H(2) and 2 moles of I(2) are taken initially in a two lit...
Text Solution
|
- Mole of PCl(5) is heated in a closed vessel of 1 litre capacity. At eq...
Text Solution
|
- The equilibrium A((g))+4B((g))hArrAB(4(g)) is attained by mixing equal...
Text Solution
|
- The equilibrium between, gaseous isomers A, B and C can be represented...
Text Solution
|
- The equilibrium A(g) +4B(g) hArr AB4(g) is attained by mixing equal mo...
Text Solution
|
- Two moles of N2 and two moles of H2 are taken in a closed vessel of 5 ...
Text Solution
|