Similar Questions
Explore conceptually related problems
Recommended Questions
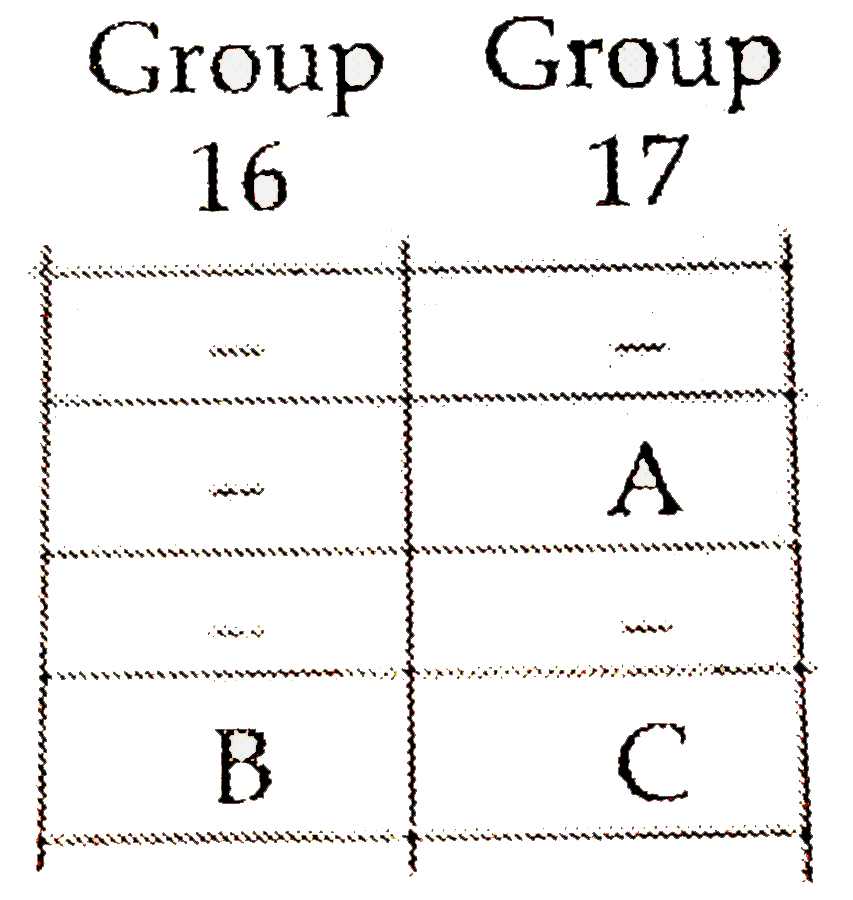
- The positions of three elements A,B and C in the periodic table are sh...
Text Solution
|
- The position of three elements A, B and C in the Periodic Table are sh...
Text Solution
|
- The positions of three elements A,B and C in the periodic table are sh...
Text Solution
|
- The position of three elements A, B and C in che periodic table are sh...
Text Solution
|
- The position of three elements A, B and C in the periodic Table is sho...
Text Solution
|
- The position of three elements A, B and C in the Periodic Table are sh...
Text Solution
|
- The position of three elements A, B and C in the Periodic Table are sh...
Text Solution
|
- The position of three elements A, B and C in the Periodic Table are sh...
Text Solution
|
- The position of three elements A, B and C in the periodic Table is sho...
Text Solution
|