Similar Questions
Explore conceptually related problems
Recommended Questions
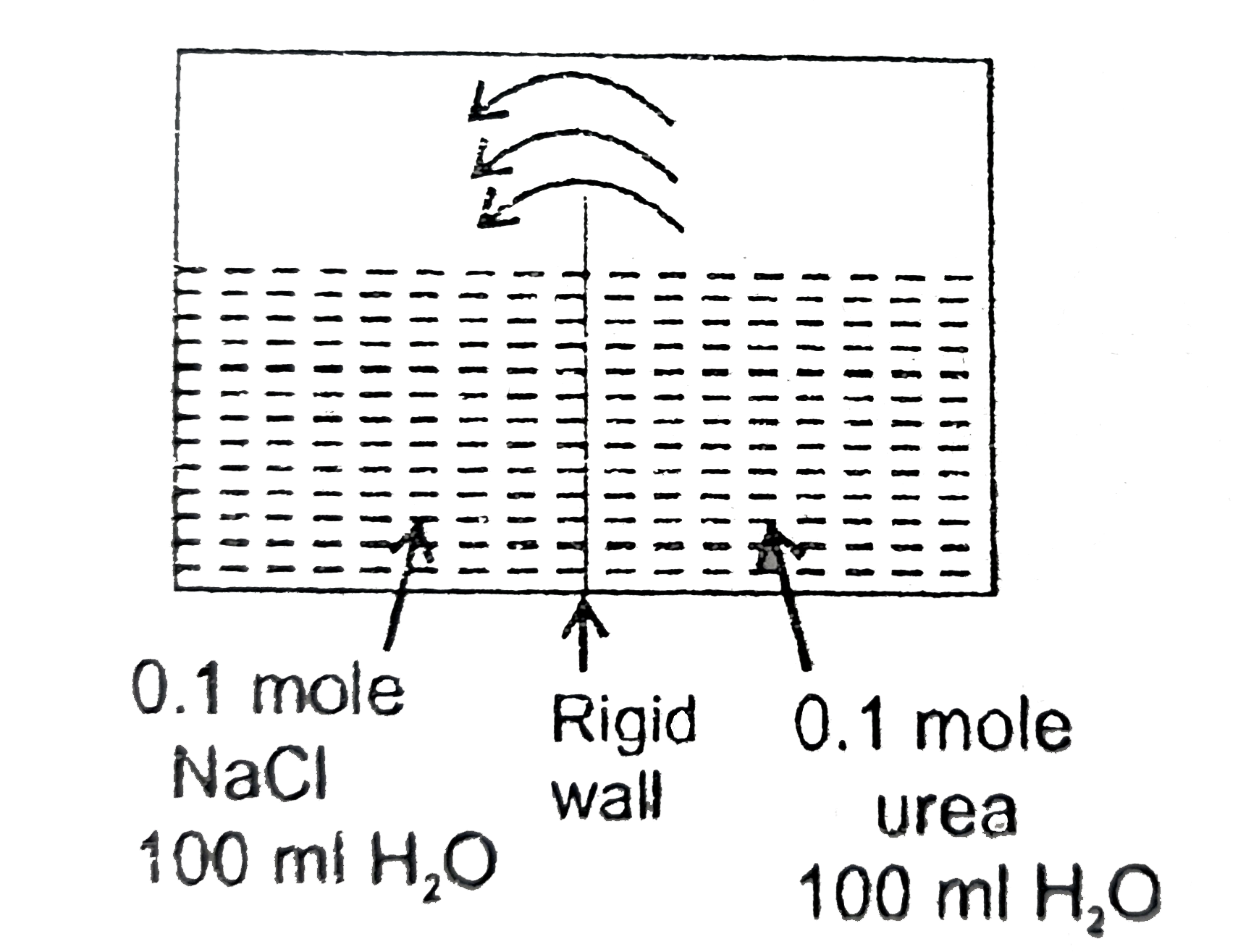
- What is the final volume of both container.
Text Solution
|
- A liquid is in equilibrium with its vapour in a sealed container at a ...
Text Solution
|
- What is the final volume of both container.
Text Solution
|
- A container contains air above liquid water. Total pressure was 800 to...
Text Solution
|
- A liquid is in equilibrium with its vapur in a sealed container at a f...
Text Solution
|
- एक द्रव सीलबंद पात्र में निम्नलिखित ताप पर इसके वाष्प से साथ ...
Text Solution
|
- A liquid is in equilibrium with its vapour in a sealed container at a ...
Text Solution
|
- A liquid is in equilibrium with its vapour in a sealed container at a ...
Text Solution
|
- In a process on a system in closed container, the initial pressure and...
Text Solution
|