Similar Questions
Explore conceptually related problems
Recommended Questions
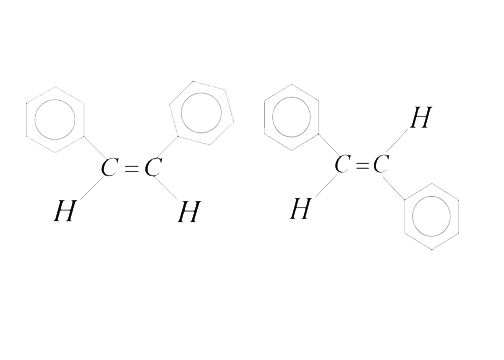
- How many statements are true for the following pair of compounds ? ...
Text Solution
|
- Assertion: Trans isomers are more stable than cis isomers Reason: Th...
Text Solution
|
- Cis and trans isomers generally
Text Solution
|
- S-I: The boiling point of cis-1,2-dichloroethene is higher than corres...
Text Solution
|
- Assertion : Boiling points of cis-isomers are higher than trans - isom...
Text Solution
|
- Assertion. Boiling points of cis-isomers are higher than those of tran...
Text Solution
|
- Assertion : Boiling points of cis-isomers are higher than trans - isom...
Text Solution
|
- (A) The boiling point of cis-1,2-dichloroethene is higher than corresp...
Text Solution
|
- How many statements are true for the following pair of compounds ? ...
Text Solution
|