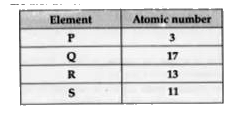
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC:2- SHORT ANSWER TYPE QUESTIONS-II|45 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC:2- LONG ANSWER TYPE QUESTIONS|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC:2- VERY SHORT ANSWER TYPE QUESTIONS|18 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|16 VideosSOLVED PAPER SSLC KARNATAKA APRIL 2019
OSWAAL PUBLICATION|Exercise QUESTIONS|49 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-TOPIC:2-SHORT ANSWER TYPE QUESTIONS-I
- How would the tendency to lose electrons change as you go i] from le...
Text Solution
|
- How does the atomic radius change as we go : (i) from left to right ...
Text Solution
|
- (i) State the basis of classification of elements in the modern period...
Text Solution
|
- How does metallic character of elements vary on moving from : (i) Le...
Text Solution
|
- The atomic radii of three elements A, B and C of a periodic table are ...
Text Solution
|
- (i) Atomic number of Mg and Al are 12 and 13, respectively. Write down...
Text Solution
|
- Arrange the following elements in the descending order of atomic size ...
Text Solution
|
- Three elements X, Y and Z belong to 17^(th) group but 2^(nd),3^(rd)and...
Text Solution
|
- i] Atomic radius of hydrogen is 37 pm. Express it in metres. ii] How...
Text Solution
|
- Nitrogen (atomic number 7) and phosphorous (atomic number 15) belong t...
Text Solution
|
- Na, Mg, Al and P belong to 3^(rd) period but are placed in first, seco...
Text Solution
|
- (i) Element 'Y' with atomic number 3 combines with element 'A' with at...
Text Solution
|
- (i) How does the tendency to gain electrons change as we go down the g...
Text Solution
|
- Given below are atomic radii of same elements of second period. {:("...
Text Solution
|
- Out of the elements X and Y, which has bigger atomic radius? Give reas...
Text Solution
|
- Two elements X and Y have atomic numbers 12 and 16 respectively. To wh...
Text Solution
|
- The atomic number of elements A, B, C, D and E are given below: F...
Text Solution
|
- The position of three elements X, Y and Z in the periodic table are sh...
Text Solution
|
- (i) An element has electronic configuration 2, 8, 6. Explain its posit...
Text Solution
|
- Some of the elements and their atomic numbers are mentioned in the fol...
Text Solution
|