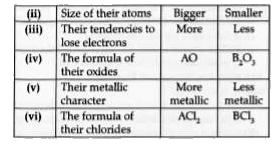
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC:2- LONG ANSWER TYPE QUESTIONS|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise NCERT CORNER -INTEXT QUESTIONS|13 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC:2-SHORT ANSWER TYPE QUESTIONS-I|25 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|16 VideosSOLVED PAPER SSLC KARNATAKA APRIL 2019
OSWAAL PUBLICATION|Exercise QUESTIONS|49 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-TOPIC:2- SHORT ANSWER TYPE QUESTIONS-II
- Name any two elements of group one and write their electronic configur...
Text Solution
|
- An element 'M' with electronic configuration (2,8,2) combines separate...
Text Solution
|
- Two elements P and Q belong to the 3rd period of the Modern Periodic T...
Text Solution
|
- An element 'X' (Atomic number = 20) burns in the presence of oxygen to...
Text Solution
|
- An element 'X' belongs to third period and second group of the Modern ...
Text Solution
|
- The atomic number of an element 'X' is 19. (i) Write its electronic ...
Text Solution
|
- An element 'X' belongs to the 3rd period and group 16 of the Modern Pe...
Text Solution
|
- An element X has mass number 35 and number of neutrons = 18. Write ato...
Text Solution
|
- Three elements 'X', 'Y' and 'Z' have atomic numbers 7, 8 and 9 respect...
Text Solution
|
- The position of eight elements in the modern periodic table is given b...
Text Solution
|
- Four elements A, B, C and D have atomic numbers 12, 13, 14 and 15 resp...
Text Solution
|
- From the following elements : (4)Be,(9)F,(19)K,(20)Ca (i) Select t...
Text Solution
|
- Na, Mg and Al are the elements of the same period of modern periodic t...
Text Solution
|
- The elements (4)Be,(12)Mgand(20)Ca each having two, valence electrons ...
Text Solution
|
- Two elements 'P' and 'Q' belong to the same period of the modern perio...
Text Solution
|
- The atomic number of an element 'X' is 20. (i) Determine the positio...
Text Solution
|
- Four elements P, Q, R and S belong to the third period of the Modern P...
Text Solution
|
- In the following table, the positions of six elements A, B, C, D, E an...
Text Solution
|
- The electrons in the atoms of four elements A, B, C and D are distribu...
Text Solution
|
- Based on the group valency of elements state the formula for the follo...
Text Solution
|