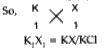Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC:2- LONG ANSWER TYPE QUESTIONS|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise NCERT CORNER -INTEXT QUESTIONS|13 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC:2-SHORT ANSWER TYPE QUESTIONS-I|25 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|16 VideosSOLVED PAPER SSLC KARNATAKA APRIL 2019
OSWAAL PUBLICATION|Exercise QUESTIONS|49 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-TOPIC:2- SHORT ANSWER TYPE QUESTIONS-II
- The position of eight elements in the modern periodic table is given b...
Text Solution
|
- Four elements A, B, C and D have atomic numbers 12, 13, 14 and 15 resp...
Text Solution
|
- From the following elements : (4)Be,(9)F,(19)K,(20)Ca (i) Select t...
Text Solution
|
- Na, Mg and Al are the elements of the same period of modern periodic t...
Text Solution
|
- The elements (4)Be,(12)Mgand(20)Ca each having two, valence electrons ...
Text Solution
|
- Two elements 'P' and 'Q' belong to the same period of the modern perio...
Text Solution
|
- The atomic number of an element 'X' is 20. (i) Determine the positio...
Text Solution
|
- Four elements P, Q, R and S belong to the third period of the Modern P...
Text Solution
|
- In the following table, the positions of six elements A, B, C, D, E an...
Text Solution
|
- The electrons in the atoms of four elements A, B, C and D are distribu...
Text Solution
|
- Based on the group valency of elements state the formula for the follo...
Text Solution
|
- Consider two elements ‘A’ (Atomic number 17) and 'B' (Atomic number 19...
Text Solution
|
- Study the following table in which positions of six elements A, B, C, ...
Text Solution
|
- The elements Li, Na and K, each having one valence electron, are in pe...
Text Solution
|
- An atom has electronic configuration 2, 8, 2. (i) What is the atomic...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 respectively in the same ...
Text Solution
|
- Four elements A, B, C and D along with their electronic configuration ...
Text Solution
|
- Four elements A, B, C and D have atomic numbers 12, 13, 14 and 15 resp...
Text Solution
|
- Three elements X, Y and Z have atomic numbers 7, 10 and 14 respectivel...
Text Solution
|
- From the following part of the periodic table, answer the following qu...
Text Solution
|