Similar Questions
Explore conceptually related problems
Recommended Questions
- Solubility of oxygen gas in water follows Henry's law. When the solubi...
Text Solution
|
- According to William Henry,the solubility of a gas in liquid depends o...
Text Solution
|
- Molar solubility of helium, nitrogen and oxygen are plotted against pa...
Text Solution
|
- Solubility of oxygen gas in water follows Henry's law. When the solubi...
Text Solution
|
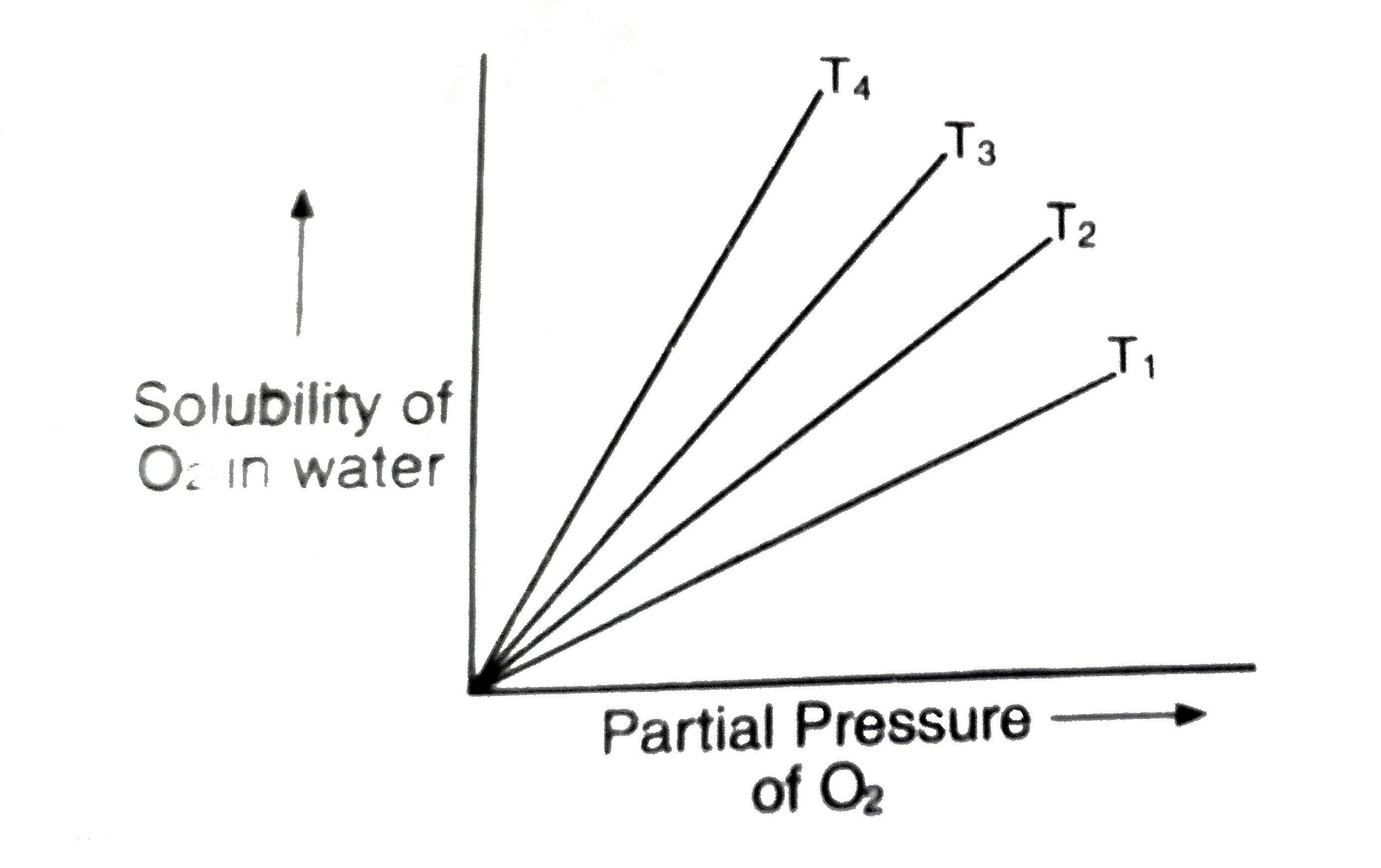
- State Henry's law. What is the effect of temperature on the solubility...
Text Solution
|
- State Henry's law. How does solubility of a gas in water varies with t...
Text Solution
|
- At 293 K temperature, if partial pressure of all given gases are same,...
Text Solution
|
- Assertion. Greater the value of Henry's constant of a gas in a particu...
Text Solution
|
- Plot a graph with respect to Henry's law stating the solubility of HCl...
Text Solution
|