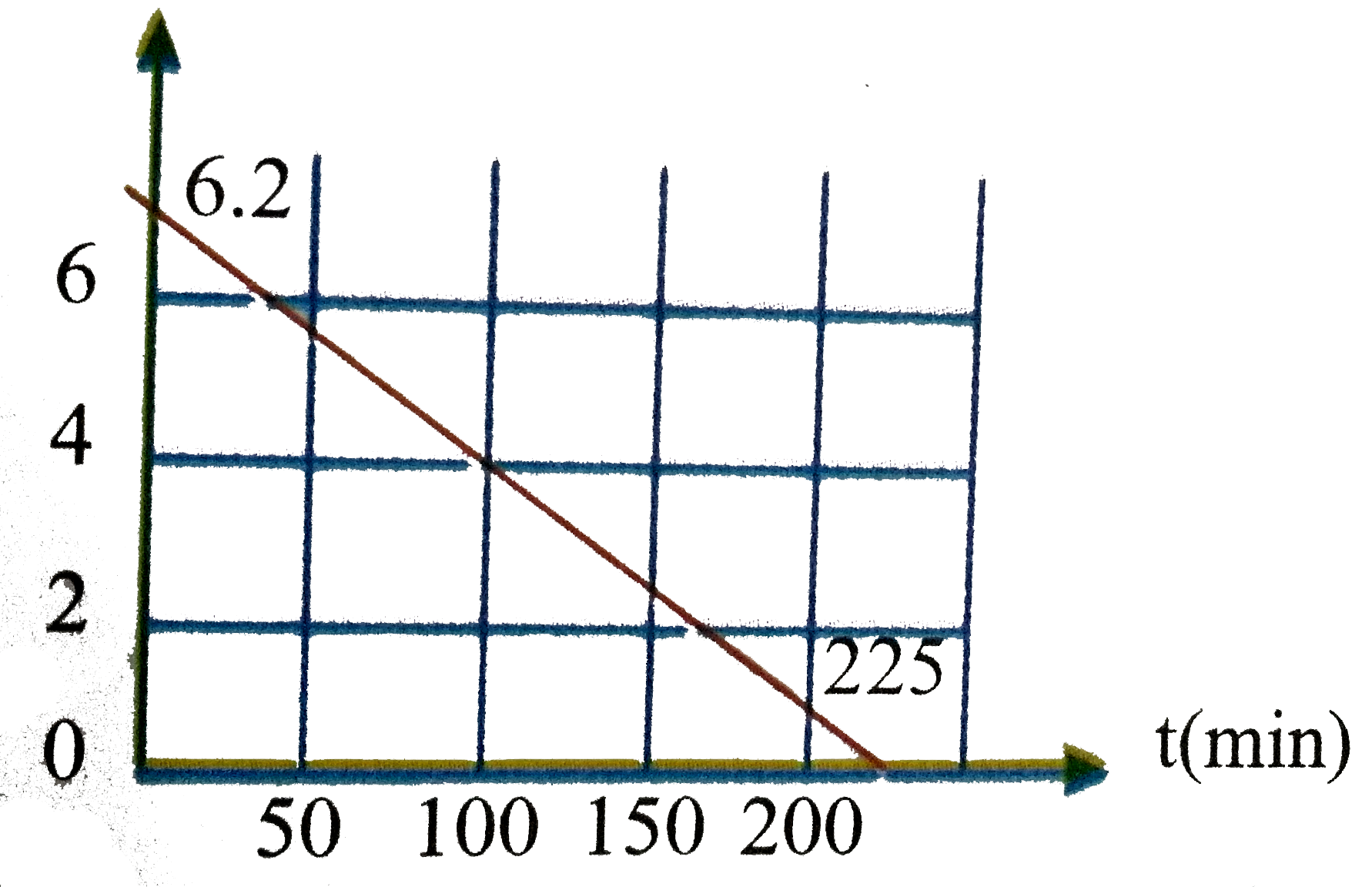
Similar Questions
Explore conceptually related problems
Recommended Questions
- The table that follows shows some measurementf of the decay rate of a ...
Text Solution
|
- The rate of radioactive decay of a sample are 3 xx 10^(8) dps and 3 xx...
Text Solution
|
- A certain nuclide has a half life period of 30 min. If a sample contai...
Text Solution
|
- A radio nuclide A(1) with decay constant lambda(1) transforms into a r...
Text Solution
|
- The table that follows shows some measurementf of the decay rate of a ...
Text Solution
|
- The count rate of a Geiger Muller counter for the radiation of a radio...
Text Solution
|
- The half life of a radio nuclide is 77 days then its decay constant is
Text Solution
|
- Derive an expression for the half-life of a radio active nuclide.
Text Solution
|
- The half life of 2g sample of radioactive nuclide 'X' is 15 min. The h...
Text Solution
|