Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYANMICS
KUMAR PRAKASHAN|Exercise Section-C (Objective Questions (VSQs))|45 VideosTHERMODYANMICS
KUMAR PRAKASHAN|Exercise Section-C (Objective Questions (True & False ))|8 VideosTHERMODYANMICS
KUMAR PRAKASHAN|Exercise Section-B Numericals (Numerical From Textual Exercise)|10 VideosTHERMAL PROPERTIES OF MATTER
KUMAR PRAKASHAN|Exercise Question Paper (Section - D) (Answer following in brief :) Each carry 4 marks|1 VideosUNITS AND MEASUREMENT
KUMAR PRAKASHAN|Exercise Section -F (Questions from Module )|20 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-THERMODYANMICS -Section-B Numericals (Numerical From .Darpan. Based on Textbook )
- A gaseous system absorbs 450 cal heat. The work done by the system is ...
Text Solution
|
- The heat capacity of a silver coin is 1.128 "cal" .^@C^(-1), What shou...
Text Solution
|
- An ideal gas is enclosed in a closed container of 0.0083 m^3 at 300 K ...
Text Solution
|
- Calculate the work required to be done to increase the temperature of ...
Text Solution
|
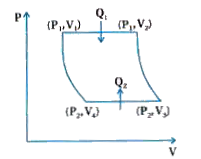
- A cyclic process consisting of two isobaric and two adiabatic processe...
Text Solution
|
- The temperature of the sink of a carnot engine is 300 K and its effici...
Text Solution
|
- ab and bc curves in the figure represent an isothermal and an adiabati...
Text Solution
|
- In a carnot engine, temperature of the source is 500 K and that of the...
Text Solution
|
- (a) How much heat should be provided to ice of 720 g mass, lying at -1...
Text Solution
|
- 1 mole of an ideal gas at 27^@ C temperature and 2 atm pressure is com...
Text Solution
|
- For an adiabatic process PV^gamma = constant. Evaluate this "constant...
Text Solution
|