A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-BEHAVIOUR OF PERFECT GAS AND KINETIC THEORY-CHECK YOUR NEET VITALS
- About 0.014 kg nitrogen is enclosed in a vessel at temperature of 27^...
Text Solution
|
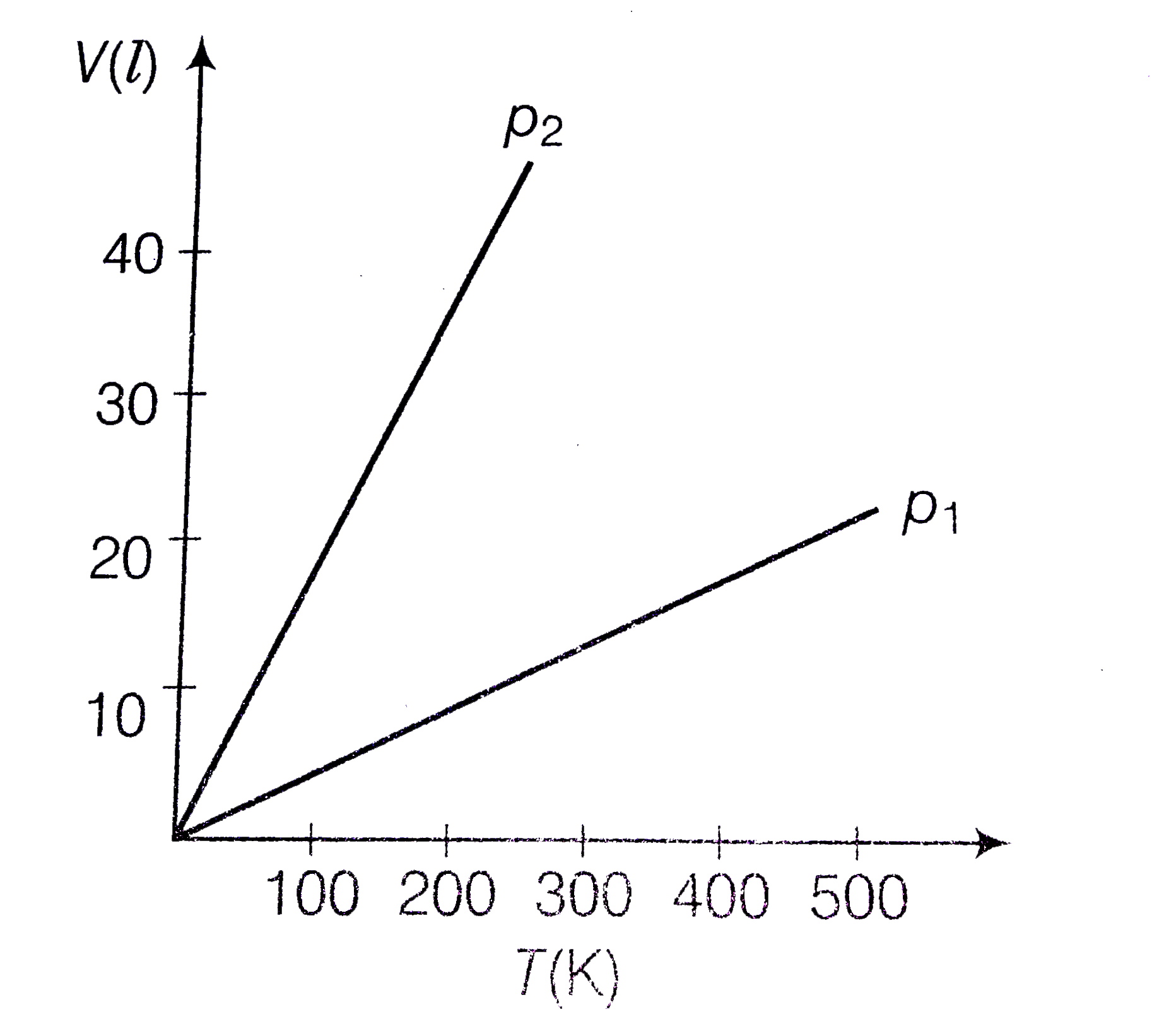
- Volume versus temperature graphs for a given mass of an ideal gas are ...
Text Solution
|
- A real gas behaves like an ideal gas if its
Text Solution
|
- Which of the following graphs represent the behaviour of an ideal gas ...
Text Solution
|
- Pressure of a gas at constant volume is proportional to
Text Solution
|
- Three moles of oxygen ar mixed with two moles of helium. What will be ...
Text Solution
|
- A vessel contains two non-reactive gases neon (monoatomic) and oxygen ...
Text Solution
|
- If C(p) and C(v) denoted the specific heats of unit mass of nitrogen a...
Text Solution
|
- A vessel is filled with a gas at a pressure of 76 cm of mercury at a c...
Text Solution
|
- Two moles of gas A at 27^(@)C mixed with a 3 moles of gas at 37^(@)C. ...
Text Solution
|
- When the temperature of a gas filled in a closed vessel is increased b...
Text Solution
|
- (1//2) mole of helium is contained in a container at STP how much heat...
Text Solution
|
- One mole of an ideal monoatomic gas at temperature T(0) expands slowel...
Text Solution
|
- The average kinetic energy of O(2) at a particular temperatures is 0.7...
Text Solution
|
- If a gas has 5 degrees of freedom ratio of specific heats of gas 5
Text Solution
|
- The volume of water molecule is Take, density of wter is 10^(3) kg m...
Text Solution
|
- When an ideal gas is compressed adiabatically, is temperature rises th...
Text Solution
|
- The molecules of a given mass of gas have root mean square speeds of 1...
Text Solution
|
- Which one of the following is/are assumptions of kinetic theory of gas...
Text Solution
|
- The kinetic theory of gases gives the formula PV=(1)/(3)Nmv^(bar2) for...
Text Solution
|