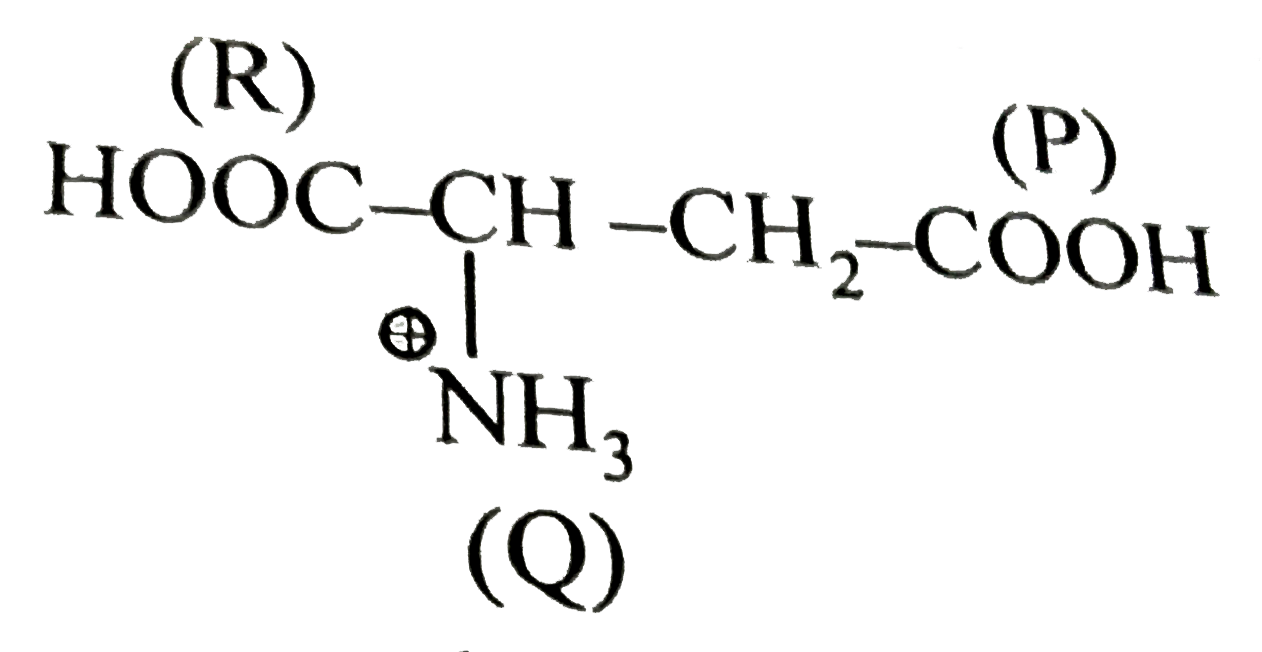Similar Questions
Explore conceptually related problems
Recommended Questions
- The pKa values for the three acidic group P,Q,R are 4.3, 9.7 and 2.2 r...
Text Solution
|
- The pK(a1) and pK(a2) of an amino acid are 2.3 and 9.7 respectively. T...
Text Solution
|
- The pKa values for the three acidic group P,Q,R are 4.3, 9.7 and 2.2 r...
Text Solution
|
- At the isoelectric point, amino acids are present as
Text Solution
|
- The pka values for the three ionizable groups x, y & z of glutamic aci...
Text Solution
|
- What is amino acid ?
Text Solution
|
- At isoelectric point, amino acid is present as
Text Solution
|
- The Pka values for 3-Ionisable groups x, y, z of glytamic acid are 4.3...
Text Solution
|
- The pK(al) and pK(a2) of an amino acid are 2.3 and 9.7 respectively. ...
Text Solution
|