Similar Questions
Explore conceptually related problems
Recommended Questions
- What will be the work done in process CB for 1 mol of an ideal gas if ...
Text Solution
|
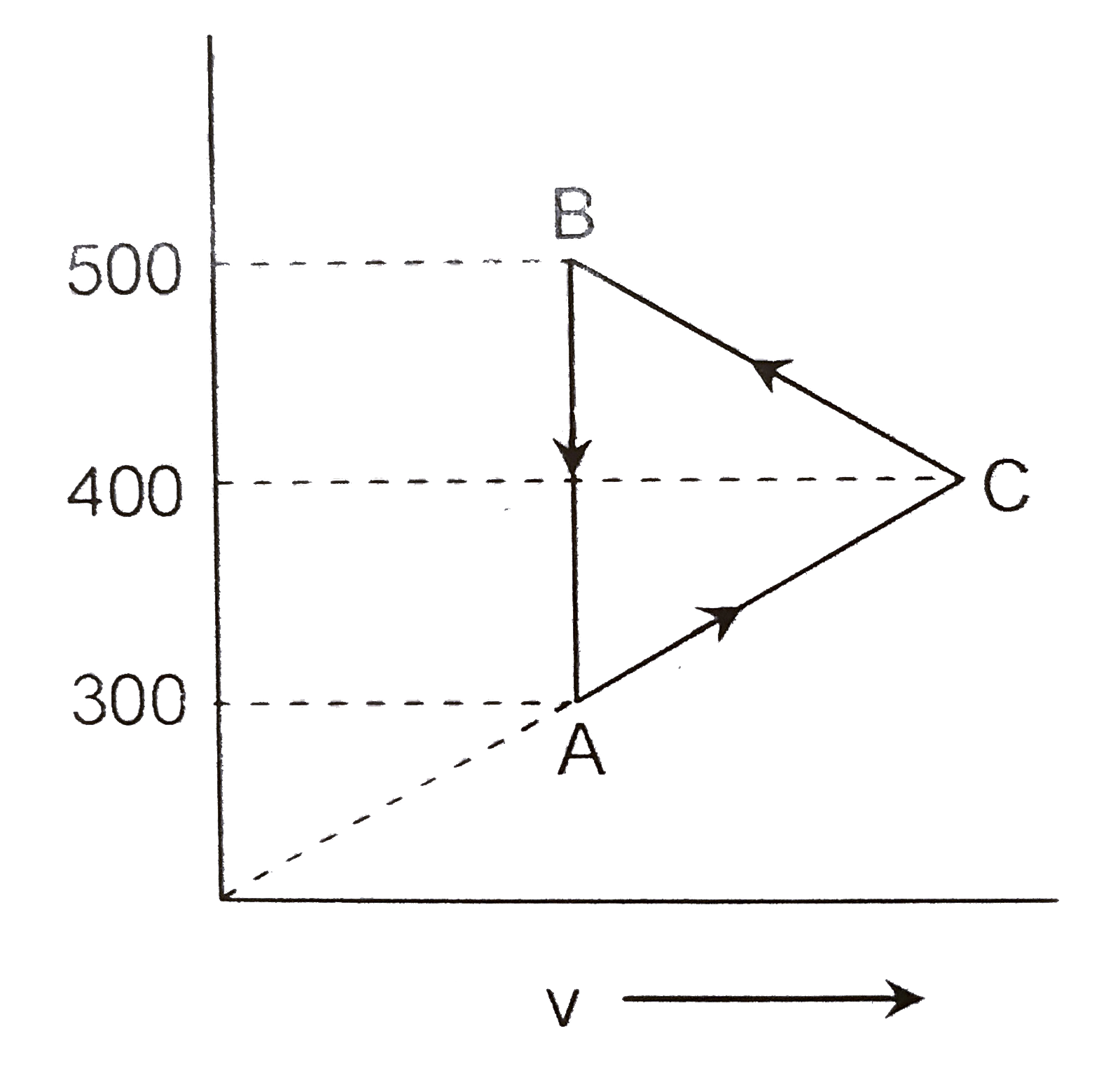
- Consider the cyclic process ABCA, shown in, performed on a sample of ...
Text Solution
|
- In isobaric process of ideal gas (f=5) work done by gas is equal of 10...
Text Solution
|
- In a process, the pressure of an ideal gas is proportional to square o...
Text Solution
|
- What will be the work done in process CB for 1 mol of an ideal gas if ...
Text Solution
|
- For 1 mol of an ideal gas work in process AB will be:
Text Solution
|
- Calculate work done is process BC for 1 mol of an ideal gas if total 6...
Text Solution
|
- An ideal gas system undergoes an isothermal process, then the work don...
Text Solution
|
- In a thermodynamic process on an ideal diatomic gas, work done by the ...
Text Solution
|