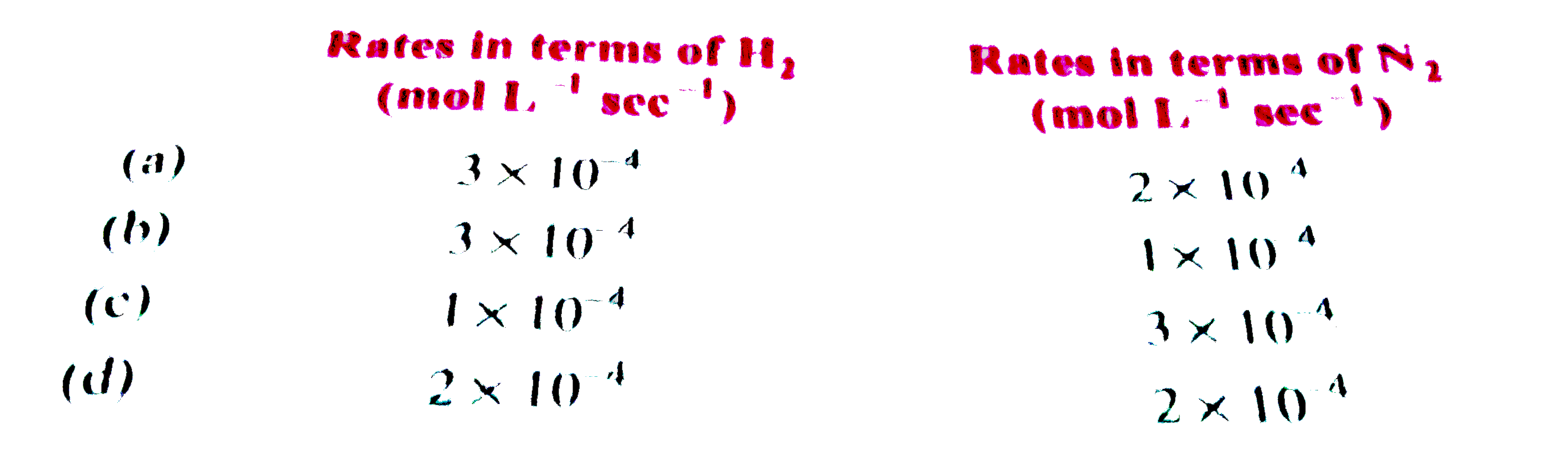Similar Questions
Explore conceptually related problems
Recommended Questions
- In the hober's process of ammonia manufacture, N(2)(g)+3H(2)(g) rarr...
Text Solution
|
- In a reaction N(2(g))+3H(2(g))to2NH(3(g)) rate of appearance of NH(3) ...
Text Solution
|
- For this reaction N(2(g))+3H(2(g))to2NH(3(g)) The relation between (d(...
Text Solution
|
- Conside the chemical reaction, N(2)(g) + 3H(2)(g)rarr2NH(3)(g) The...
Text Solution
|
- In the hober's process of ammonia manufacture, N(2)(g)+3H(2)(g) rarr...
Text Solution
|
- Conisder the chemical reaction N(2)(g) + 3H(2)(g) rarr 2NH(3)(g) T...
Text Solution
|
- In a catalytic experiment involving Haber's process, N(2)(g)+3" H"(2)(...
Text Solution
|
- For the reaction : N(2)(g)+3H(2)(g)rarr2NH(3)(g) . If rate of appearan...
Text Solution
|
- The commercial poduction of ammonia is represented by the equation, N(...
Text Solution
|