Similar Questions
Explore conceptually related problems
Recommended Questions
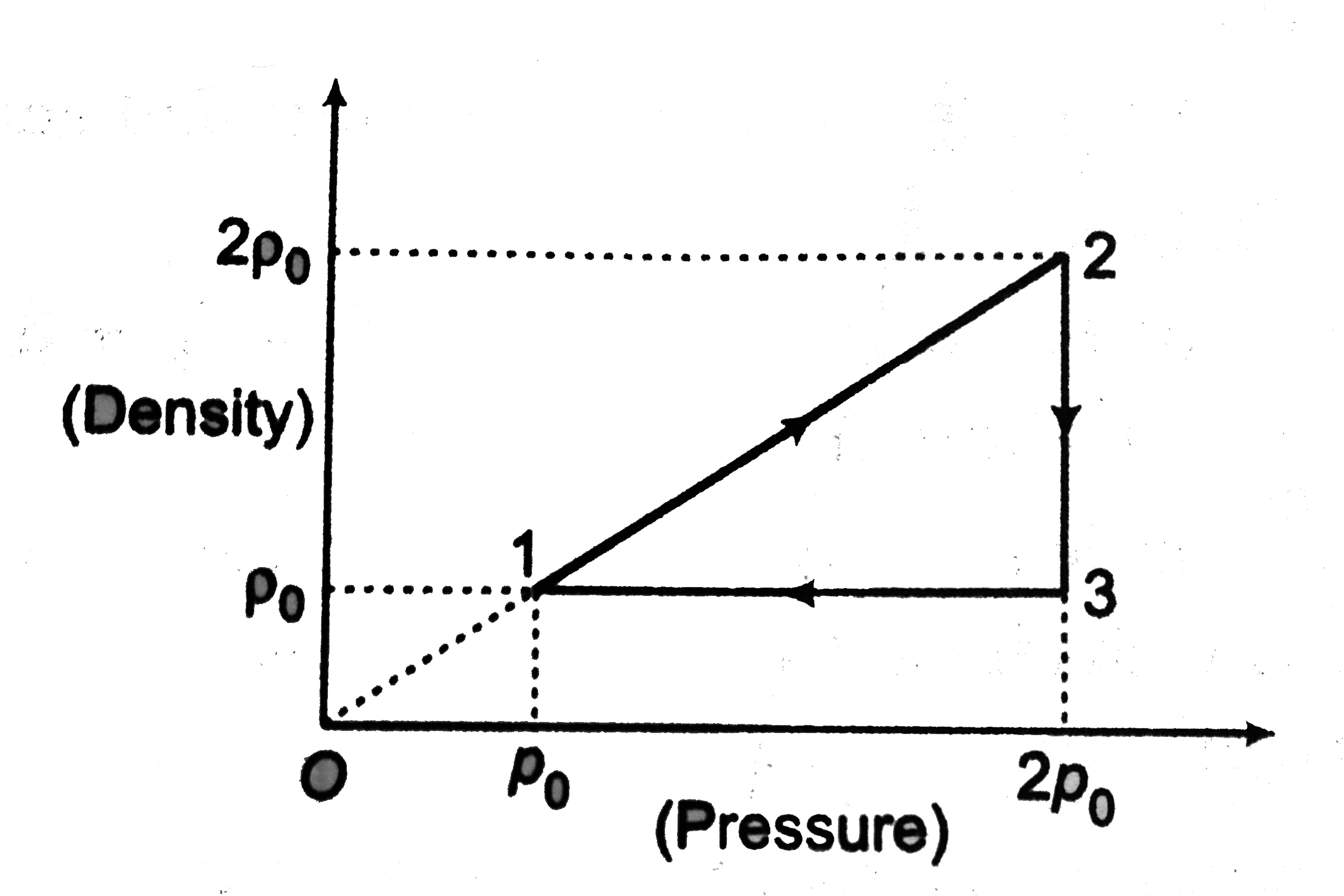
- The density (rho) versus pressure (p) graph of one mole of an ideal mo...
Text Solution
|
- The density (rho) versus pressure (p) graph of one mole of an ideal mo...
Text Solution
|
- Two moles of a monatomic ideal gas undergo a cyclic process ABCDA as s...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a process as shown in th...
Text Solution
|
- One mole of an ideal gas is taken through the cyclic process ABCDA, as...
Text Solution
|
- A cyclic process performed on one mole of an ideal gas. A total 1000 J...
Text Solution
|
- One mole of a mono atomic gas of molar mass M undergoes a cyclic proce...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|