A cylindrical container of volume `V_(0)` is divided into two parts by a thin conducting separator of negligible mass. The walls of the container are adiabatic. Ideal gases are filled in the two parts such that the pressures are `P_(0) " and " 2 P_(0)` when the separator is held in the middle of the container (see figure). Now the separator is slowly slid and released in a position where it stays in equilibrium. Find the volume of the two parts.
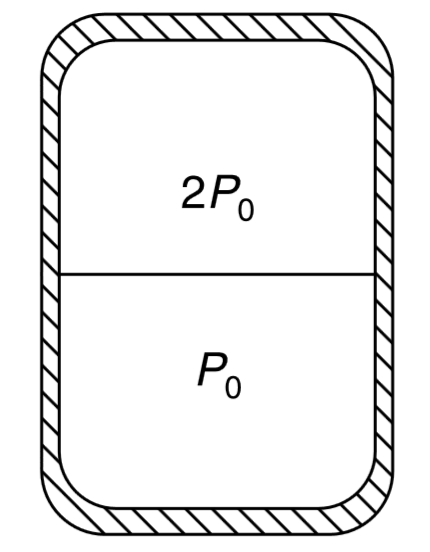
A cylindrical container of volume `V_(0)` is divided into two parts by a thin conducting separator of negligible mass. The walls of the container are adiabatic. Ideal gases are filled in the two parts such that the pressures are `P_(0) " and " 2 P_(0)` when the separator is held in the middle of the container (see figure). Now the separator is slowly slid and released in a position where it stays in equilibrium. Find the volume of the two parts.


Similar Questions
Explore conceptually related problems
Show a cylindrical tube of volume V_(0) divided in two parts by a frictionless separator. The walls of the tube are adiabatic but the separator is conducting, Ideal gases are filled in the two parts. When the separator is kept in the middle, the pressure are p_(1) and p_(2) in the left part and the right part respectively. The separator is slowly slid and is released at a position where it can stay in equilibrium. Find the volumes if the two parts.
Show a cylindrical tube of volume V_(0) divided in two parts by a frictionless separator. The walls of the tube are adiabatic but the separator is conducting, Ideal gases ate filled in the two parts. When the swparator is kept in the middle, the pressure are p_(1) and p-(2) in the left part and the right part respectively. The separator is slowly slid and is released at a position where it can stay in equilibrium. Find the volumes if the two parts. (figure)
In given figure, an adiabatic cylindrical tube of volume 2V_(0) is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure P_(1) and temperature T_(1) where as in the right side having pressure P_(2) and temperature T_(2).C_(p)//C_(v) =gamma is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the final volumes of the two parts (b) the heat given to the gas in the left part and (c) the final common pressure of the gases,
In given figure, an adiabatic cylindrical tube of volume 2V_(0) is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure P_(1) and temperature T_(1) where as in the right side having pressure P_(2) and temperature T_(2).C_(p)//C_(v) =gamma is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the final volumes of the two parts (b) the heat given to the gas in the left part and (c) the final common pressure of the gases,
In given figure, an adiabatic cylindrical tube of volume 2V_(0) is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure P_(1) and temperature T_(1) where as in the right side having pressure P_(2) and temperature T_(2).C_(p)//C_(v) =gamma is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the final volumes of the two parts (b) the heat given to the gas in the left part and (c) the final common pressure of the gases,
Figure shows an adiabatic cylindrical tube of volume (V_0) divided in two parts by a frictionless adiabatic separator . Initially, the separator is kept in the middle, an ideal gas at pressure (P_1) and the temperatures (T_1) is injected into the left part and the another ideal gas at pressures (P_2) and temperature (T_2) is injected into the right part. (C_p / C_v = gamma) is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the volumes of the parts, (b) the heat given to the gas in the left part and (c) the final common pressure of the gases.
Figure shows an adiabatic cylindrical tube of volume (V_0) divided in two parts by a frictionless adibatic separator . Initially, the separator is kept in the middle, an ideal gas at pressure (p_1) and the temperatures (T_1) is injected into the left part and the another ideal gas at pressures (P_2) and temperature (T_2) is injected into the right part. (C_p / C_v = gamma) is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the volumes of the parts, (b) the heat given to the gas in the left part and (c) the final common pressure of the gases.
Following figure shows on adiabatic cylindrical container of volume V_(0) divided by an adiabatic smooth piston (area of cross-section = A ) in two equal parts. An ideal gas (C_(p)//C_(y)=lambda) is at pressure P_1 and temperature T_1 in left part and gas at pressure P_2 and temperature T_2 in right part. The piston is slowly displaced and released at a position where it can stay in equilibrium. The final pressure of the two parts will be (Suppose x = displacement of the piston)
Following figure shows on adiabatic cylindrical container of volume V_(0) divided by an adiabatic smooth piston (area of cross-section = A ) in two equal parts. An ideal gas (C_(p)//C_(y)=lambda) is at pressure P and temperature T in left part and gas at pressure P and temperature T in right part. The piston is slowly displaced and released at a position where it can stay in equilibrium. The final pressure of the two parts will be (Suppose x = displacement of the piston)
An insulting container of volume 2V_(0) is divided in two equal parts by a diathermic (conducting) fixed piston. The left part contains one mole of monoatomic ideal gas whereas right part contains two moles of the same gas. Initial pressue on each side is P_(0) . The system is left for sufficient time so that a steady state is reached. Find (a) the work done by the gas in the left part during the process, (b) initial temperature in the two sides, (c) the final common temperature reached by the gases, (d) the heat given to the gas in the right part and (e) the increase in the internal energy of the gas in the left part.
Recommended Questions
- A cylindrical container of volume V(0) is divided into two parts by a ...
Text Solution
|
- Show a cylindrical tube of volume V(0) divided in two parts by a frict...
Text Solution
|
- Figure shows an adiabatic cylindrical tube of volume (V0) divided in t...
Text Solution
|
- Fig. shows a horzontal cylindrical container of length 30 cm , which i...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- In given figure, an adiabatic cylindrical tube of volume 2V(0) is divi...
Text Solution
|
- A cylindrical container of volume V(0) is divided into two parts by a ...
Text Solution
|