Similar Questions
Explore conceptually related problems
Recommended Questions
- A L shaped container has dimensions shown in the fig. It has circular ...
Text Solution
|
- Two rods (one semi-circular and other straight) of same material and o...
Text Solution
|
- As shown in figure, a liquid of density rho is standing in sealed cont...
Text Solution
|
- A L shaped container has dimensions shown in the fig. It has circular ...
Text Solution
|
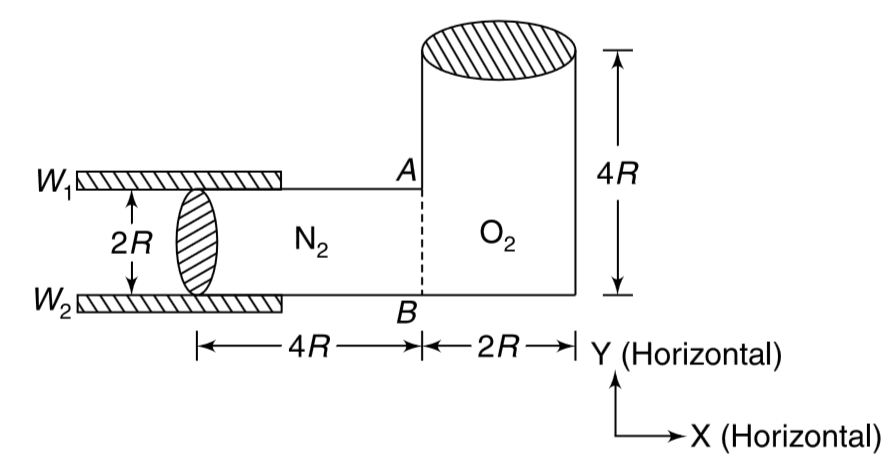
- A container is divided into two compartments. One compartment contains...
Text Solution
|
- A container is divided into two compartments. One compartment contains...
Text Solution
|
- A container of volume 1m^(3) is divided into two equal compartments by...
Text Solution
|
- Two vessels A and B of cross sections as shown contain a liquid up to ...
Text Solution
|
- A cell diagram shown below contains of one litre of buffer solution of...
Text Solution
|