Phosphorus is found in many allotropic forms, the important ones being white, red and black.
(i) White phosphorus : White phosphorus is translucent white waxy solid. It is poisonous, insoluble in water but soluble in carbon disulphide and glows in dark (chemilum inescence). It dissolves in boiling NaOH solution in an inert atmosphere giving `PH_(3)`.

`P_(4) + 3NaOH + 3H_(2)O to underset("Sodium hypophosphite")(PH_(3) + 3NaH_(2)PO_(2))`
White phosphorus is less stable and therefore more reactive than the other solid phases under normal conditions because of angular strain in `P_4` molecule where the angles are only 60°. It readily catches fire in air to give dense white fumes of `P_(4)O_(10)`
It consists of discrete tetrahedral `P_4` molecules.
(ii) Red phosphorus : When white phosphorus is heated at 573 K in an inert atmosphere for several days, Red phosphorus is obtained.
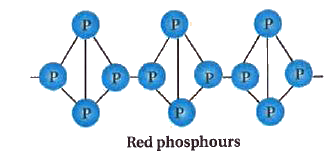
Red phosphorus is polymeric, consisting a chains of P4 tetrahedra linked together. It possess iron grey lustre and it is odourless, non-poisonous and insoluble in water as well as in carbon disulphide.
The red phosphorus when heated under high pressure, a series of phases of black phosphorus is obtained. Chemically red phosphorus is much less reactive than white phosphorus and it does not glow in dark.
(iii) Black phosphorus : Black phosphorus has two forms `alpha`-black phosphorus and `beta`-black phosphorus, `alpha`-black phosphorus is formed when red phosphorus in heated in a sealed tube at 803 K while `beta`-black phosphorus is prepared by heating white phosphorus at 473 K under high pressure.
`alpha`-block phosphorus can be sublimed in air and has opaque monoclinic or rhombohedral crystals and it does not oxidize in air. `beta`-black phosphorus burns in air upto 673 K.

