Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-A QUESTIONS (CHLORINE)|2 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-A QUESTIONS (HYDROGEN CHLORIDE)|2 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-A QUESTIONS (OXOACIDS OF SULPHUR)|1 VideosTHE D-AND F-BLOCK ELMENTS
KUMAR PRAKASHAN|Exercise Section -E MCQs asked in GUJCET/Board Exams)|50 VideosTHE SOLID STATE
KUMAR PRAKASHAN|Exercise SECTION - E (MULTIPLE CHOICE QUESTIONS)(MCQs ASKED IN BOARD EXAMS)|35 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-THE P-BLOCK ELEMENTS -SECTION-A QUESTIONS (GROUP-17 ELEMENTS (HALOGENS))
- State occurence of group-17 elements.
Text Solution
|
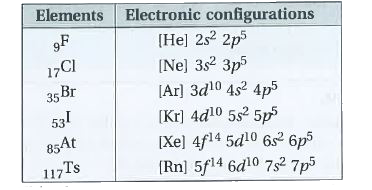
- State the electronic configurations of Group-17 elements.
Text Solution
|
- Discuss variations in atomic radii and ionization enthalpies in Haloge...
Text Solution
|
- Write a note on : (i)- Electron gain enthalpy of halogens (ii) Ele...
Text Solution
|
- Discuss physical properties of halogens.
Text Solution
|
- Write a note on oxidation states of group-17 elements.
Text Solution
|
- Explain chemical reactivity of halogens.
Text Solution
|
- Write a note on hydrogen halides.
Text Solution
|
- Explain reactivity of halogens with oxygen. OR Write a note on oxides ...
Text Solution
|
- Write a note on metal halides.
Text Solution
|
- Explain reactivity of halogens with metals.
Text Solution
|
- Write a shortnote on interhalogen compounds.
Text Solution
|
- Discuss anomalous behaviour of fluorine.
Text Solution
|