Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION -D (NCERT EXEMPLAR SOLUTION)(MATCHING THE COLUMNS)|5 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION -D (NCERT EXEMPLAR SOLUTION)(ASSERTION AND REASON TYPE)|6 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION -D (NCERT EXEMPLAR SOLUTION) (MULTIPLE CHOICE QUESTIONS MCQS (MORE THAN ONE QUESTIONS))|10 VideosTHE D-AND F-BLOCK ELMENTS
KUMAR PRAKASHAN|Exercise Section -E MCQs asked in GUJCET/Board Exams)|50 VideosTHE SOLID STATE
KUMAR PRAKASHAN|Exercise SECTION - E (MULTIPLE CHOICE QUESTIONS)(MCQs ASKED IN BOARD EXAMS)|35 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-THE P-BLOCK ELEMENTS -SECTION -D (NCERT EXEMPLAR SOLUTION) (SHORT ANSWER TYPE QUESTIONS)
- Write a balanced chemical equation for the reaction showing catalytic ...
Text Solution
|
- Write the structure of pyrophosphoric acid.
Text Solution
|
- PH3 forms bubbles when passed slowly in water but NH3 dissolves. Expla...
Text Solution
|
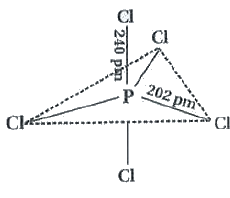
- In PCl5, phosphorus is in sffid hybridised state but all its five bond...
Text Solution
|
- Why is nitric oxide paramagnetic in gaseous state but the solid obtain...
Text Solution
|
- Give reason to explain why ClF3 exists but FCl3 does not exist.
Text Solution
|
- Out of H2O and H2S, which one has higher bond angle and why ?
Text Solution
|
- SF6 is known but SCl6 is not. Why ?
Text Solution
|
- On reaction with Cl2, phosphorus forms two types of halides 'A' and 'B...
Text Solution
|
- In the ring test of NO(3) ion, Fe^(2+) ion reduces nitrate ion to nit...
Text Solution
|
- Explain why the stability of oxoacids of chlorine increases in the ord...
Text Solution
|
- Explain why ozone is thermodynamically less stable than oxygen ?
Text Solution
|
- P4O6 reacts with water according to equation P4O6 + 6H2O to 4H3PO3. C...
Text Solution
|
- White phosphorus reacts with chlorine and the product hydrolyses in th...
Text Solution
|
- Name three oxoacids of nitrogen. Write the disproportionation reaction...
Text Solution
|
- Nitric acid forms an oxide of nitrogen on reaction with P4O10. Write t...
Text Solution
|
- Phosphorus has three allotropic forms : (i) white phosphorus (ii) ...
Text Solution
|
- Give an example to show the effect of concentration of nitric acid on ...
Text Solution
|
- PCl(5) reacts with finely divided silver on heating and a white silver...
Text Solution
|
- Phosphorus forms a number of oxoacids. Out of these oxoacids phosphini...
Text Solution
|