Similar Questions
Explore conceptually related problems
Recommended Questions
- n moles of a monoatomic gas are taken around in a cyclic process consi...
Text Solution
|
- Conider a given sample of an ideal gas (Cp / Cv = gamma ) having initi...
Text Solution
|
- n moles of a monoatomic gas are taken around in a cyclic process consi...
Text Solution
|
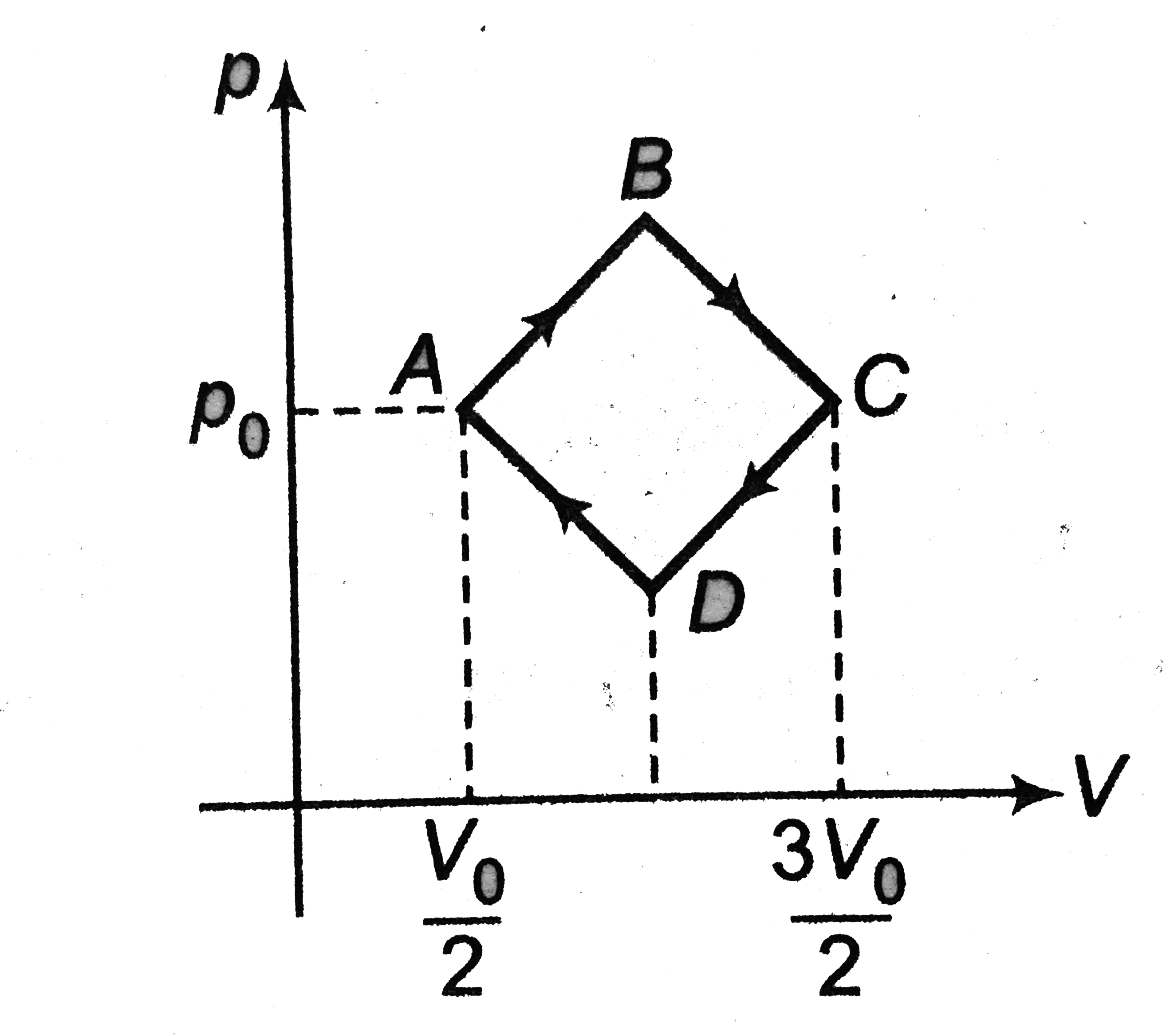
- An ideal monoatomic gas is taken round the cycle ABCDA as shown in the...
Text Solution
|
- One mole of an ideal gas passes through a process where pressure and v...
Text Solution
|
- One mole of diatomic gas undergoes a process P=(P0)/([1+(V//V0)^3]) , ...
Text Solution
|
- One mole of the ideal gas through the process p= p0 [ 1 - alpha ((V)...
Text Solution
|
- Consider that an ideal gas (n moles) is expanding in a process given b...
Text Solution
|
- Conider a given sample of an ideal gas (Cp / Cv = gamma ) having initi...
Text Solution
|