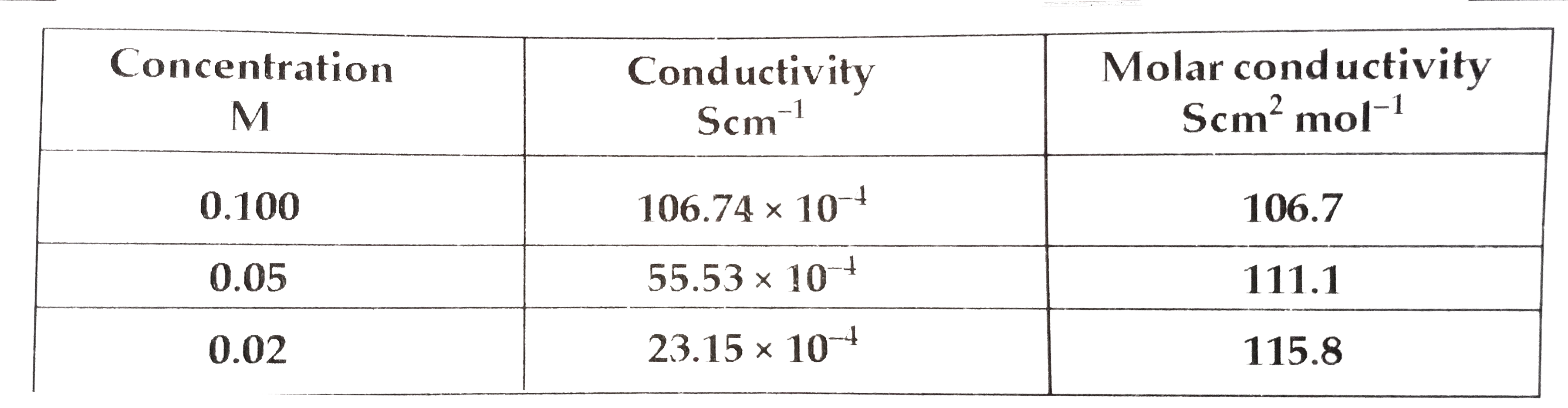Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Apply Kohlrausch law of independent migration of ions, write the e...
Text Solution
|
- For a dilute solution of a strong electrolyte, the variation of molar ...
Text Solution
|
- Define conductivity and molar conductivity for the solution of an ele...
Text Solution
|
- (a) Apply Kohlrausch law of independent migration of ions, write the e...
Text Solution
|
- KCI के 0.20 M विलयन को चालकता 298 K पर 0.0248 S cm^(-1) है । इसकी मो...
Text Solution
|
- Molar conductivity of 0.15 M solution of KCI at 298 K, if its conducti...
Text Solution
|
- If X is the conductivity of the solution and M is the molarity of the ...
Text Solution
|
- Define conductivity and molar conductivity for the solution of an elec...
Text Solution
|
- From Kohlrausch's law of independent migration of ions, how is the mol...
Text Solution
|