Similar Questions
Explore conceptually related problems
Recommended Questions
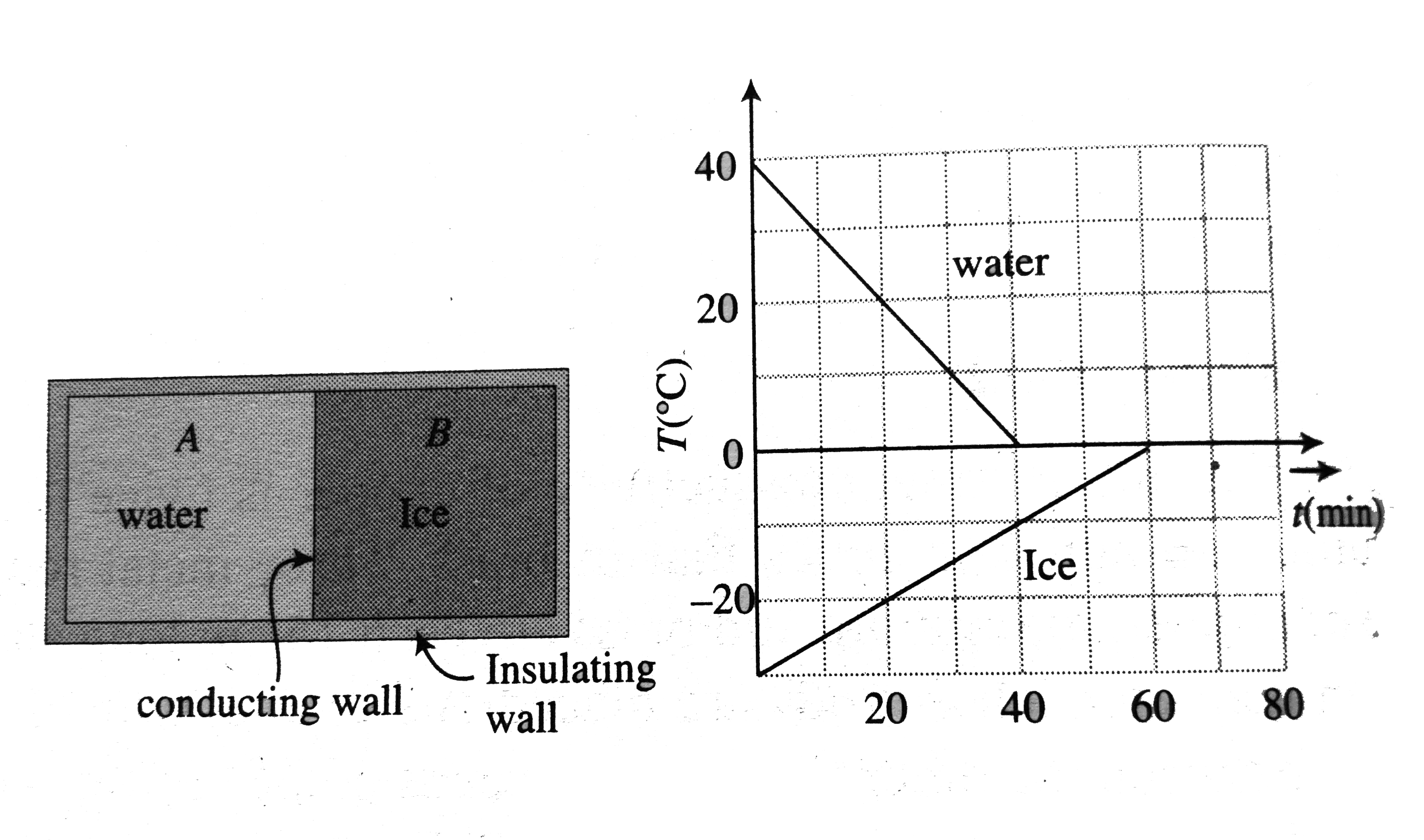
- A 0.60 kg sample of water and a sample of ice are placed in two compar...
Text Solution
|
- A 0.60 kg sample of water and a sample of ice are placed in two compar...
Text Solution
|
- A 0.60 kg sample of water and a sample of ice are placed in two compar...
Text Solution
|
- A 0.60 kg sample of water and a sample of ice are placed in two compar...
Text Solution
|
- Water at 10^(@) C is present in a thermally insulated container. Calcu...
Text Solution
|
- In an 20 m deep lake, the bottom is at a constant temperature of 4^(@...
Text Solution
|
- Pure water cooled to -15^(@)C is contained in a thermally insulated fl...
Text Solution
|
- পুকুরের জলের ওপর 5cm পুরু বরফ রয়েছে। বরফের ওপর বায়ুর তাপমাত্রা -10°C...
Text Solution
|
- (a) Two 40g ice cubes are droped into 200g of water in a thermally ins...
Text Solution
|