Similar Questions
Explore conceptually related problems
Recommended Questions
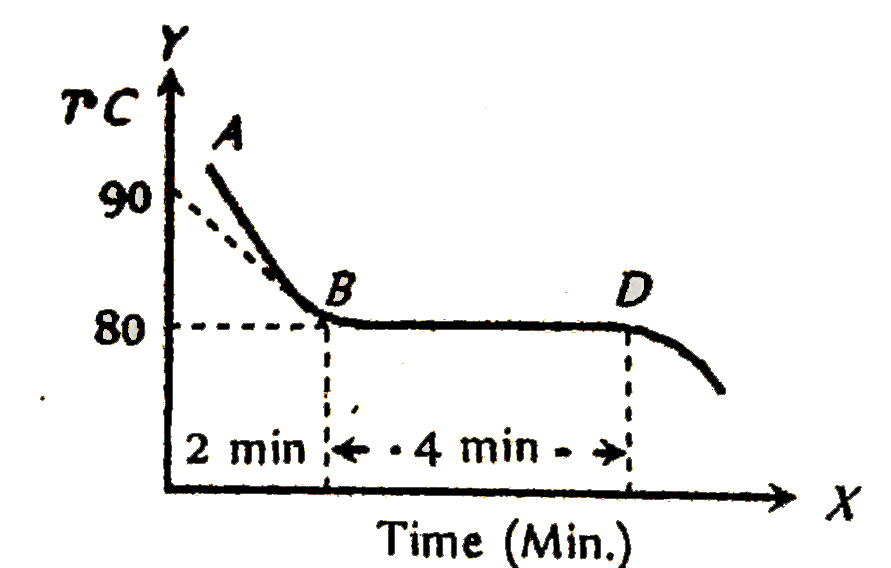
- The figure given below shows the cooling curve of pure wax material af...
Text Solution
|
- Cooling curves are drawn for three liquids a,b,c, shown in fig. for wh...
Text Solution
|
- A liquid A of specific heat 0.5 at 60^(@)C is mixed with another liqui...
Text Solution
|
- A student takes 50 g wax (specific heat =0.6 kcal//kg^(@)C ) and heats...
Text Solution
|
- Cooling graphs are drawn for three liquids a,b and c The specific heat...
Text Solution
|
- The figure given below shows the cooling curve of pure wax material af...
Text Solution
|
- Specific heat of an object : c :: Latent heat of a substance :
Text Solution
|
- Specific heat of an object : c :: Latent heat of a substance :
Text Solution
|
- Two liquids of same volume take 324s and 810s respectively in cooling ...
Text Solution
|