Similar Questions
Explore conceptually related problems
Recommended Questions
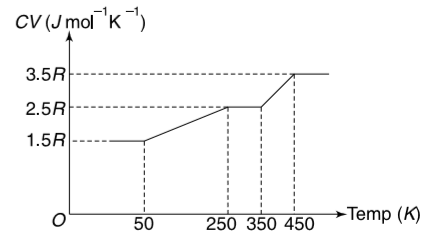
- The molar specific heat capacity at constant volume (C(V)) for an idea...
Text Solution
|
- For an ideal monoatomic gas, molar heat capacity at constant volume (C...
Text Solution
|
- The molar specific heat capacity at constant volume (C(V)) for an idea...
Text Solution
|
- One mole of an ideal gas requires 207 J of heat to raise the temperatu...
Text Solution
|
- One mole of an ideal gas requires 207J heat to raise the temperature b...
Text Solution
|
- One mole of an ideal monatomic gas requires 210 J heat to raise the te...
Text Solution
|
- One mole of an ideal gas requires 207 J heat to rise the temperature b...
Text Solution
|
- For 0.5 mol of an ideal gas, 15 cal of heat is required to raise its t...
Text Solution
|
- One mole of an ideal gas requires 207 J heat to raise its temperature ...
Text Solution
|