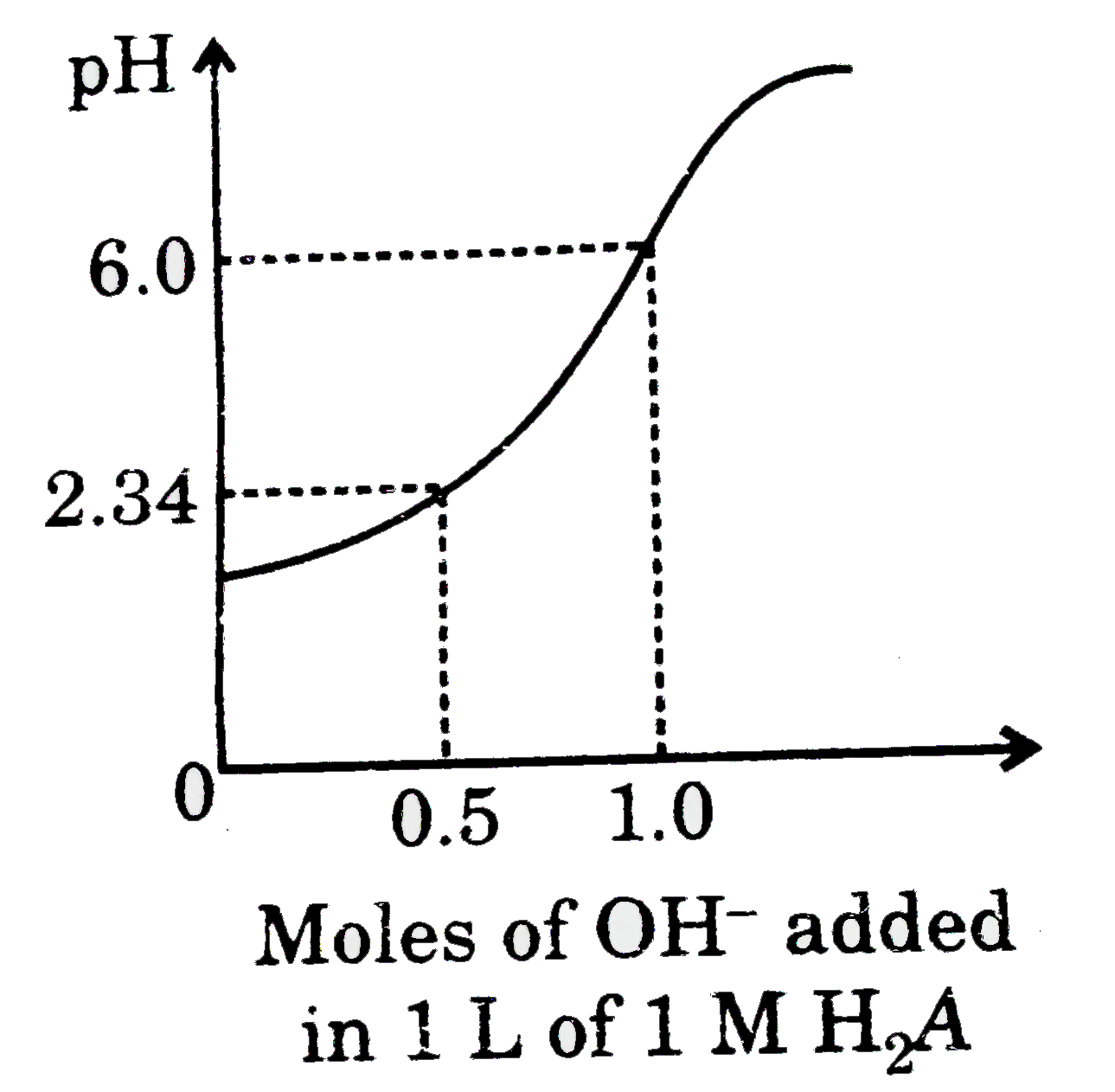Similar Questions
Explore conceptually related problems
Recommended Questions
- Titration of diprotic acid (H(2)A) by strong base has been summarised...
Text Solution
|
- Titration of diprotic acid (H(2)A) by strong base has been summarised ...
Text Solution
|
- Titration of diprotic acid (H(2)A) by strong base has been summarised ...
Text Solution
|
- Titration of diprotic acid (H(2)A) by strong base has been summarised ...
Text Solution
|
- During the titration of a weak diprotic acid (H(2)A) against a strong ...
Text Solution
|
- In the titration of a weak acid of known concentratin with a standard ...
Text Solution
|
- In the titration of weak acid against strong base match the pH of the ...
Text Solution
|
- A solution of a substances is titrated against a strong base ( or acid...
Text Solution
|
- When a strong acid is titrated using a weak base, the pH at the equiva...
Text Solution
|