Similar Questions
Explore conceptually related problems
Recommended Questions
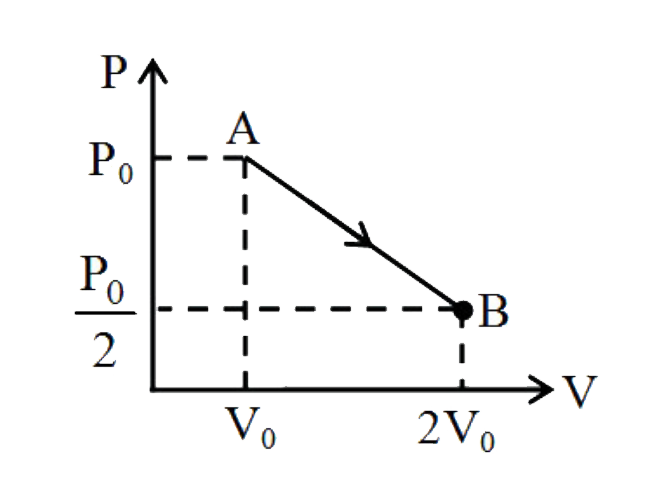
- An ideal monoatomic gas undergoes an expansion from state A to state B...
Text Solution
|
- An ideal gas undergoes isothermal expansion followed by heat removel a...
Text Solution
|
- A monoatomic ideal gas undergoes a process ABC . The heat given to the...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- If the internal energy of an ideal gas varies as U = 2PV and the gas u...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|
- An ideal gas undergoes a cyclic process as shown. Part of the process ...
Text Solution
|
- An ideal gas follows a cyclic process as shown in figure. Internal ene...
Text Solution
|
- One mole of ideal monoatomic gas is taken round the cyclic process as ...
Text Solution
|