Similar Questions
Explore conceptually related problems
Recommended Questions
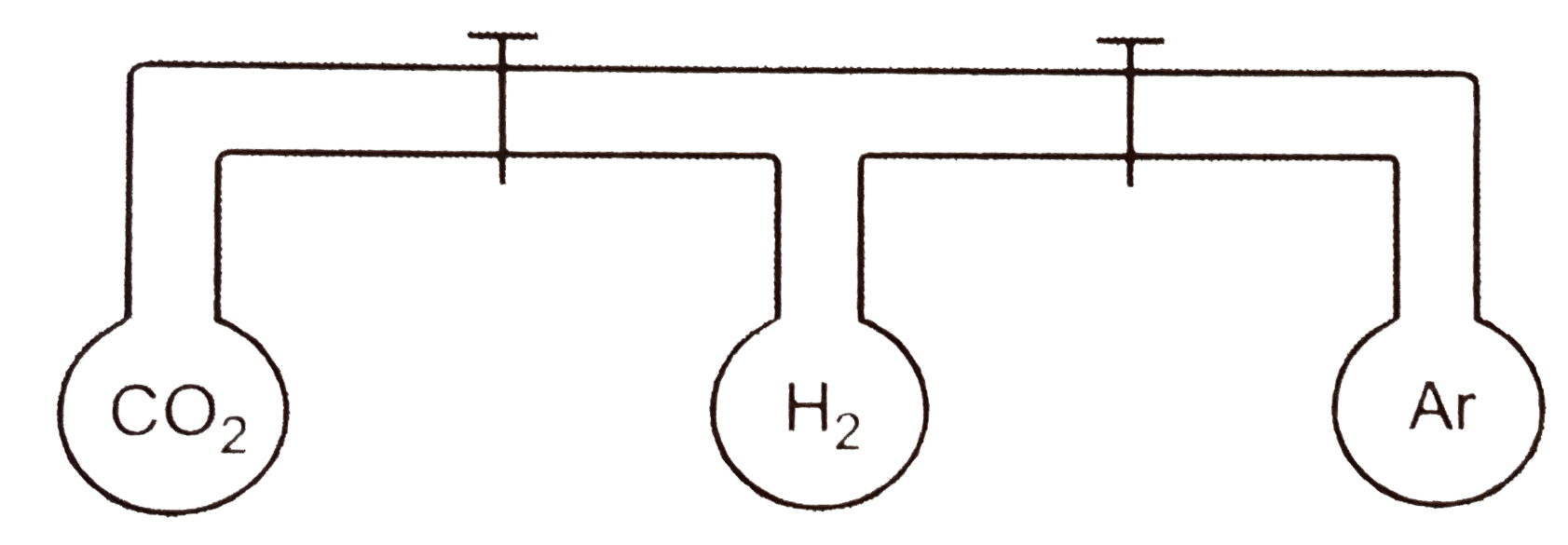
- The apparatus shown consists of three bulbs connected by stop cocks Wh...
Text Solution
|
- The apparatus shown consists of three bulbs connected by stop cocks Wh...
Text Solution
|
- Two glass bulbs A and B are connected by a very small tube having a st...
Text Solution
|
- A bulb 'X' of unknown volume containing a gas at one atmospheric press...
Text Solution
|
- The stop cock containing two bulbs of volume 5 litre and 10 litre cont...
Text Solution
|
- Two glass bulbs x and y are connected by a very small tube having a st...
Text Solution
|
- The apparatus shown consists of three bulbs connected by stopcocks of ...
Text Solution
|
- Two glass bulbs A and B are connected by a very small tube having a st...
Text Solution
|
- A bulb of unknown volume V contains a gas at 1 atm pressure. This bulb...
Text Solution
|