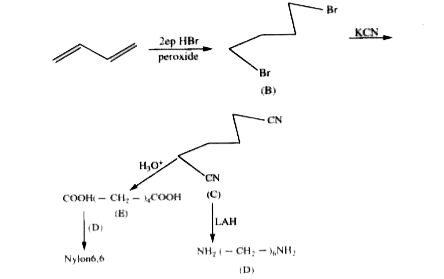
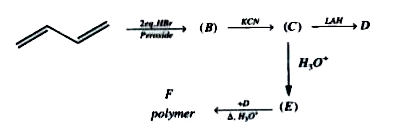A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
POLYMERS
AAKASH SERIES|Exercise Practice Sheet-1 (Match the following questions)|4 VideosPOLYMERS
AAKASH SERIES|Exercise Practice Sheet-1 (Integer answer Types Questions)|6 VideosPOLYMERS
AAKASH SERIES|Exercise Practice Sheet-1 (Single correct questions)|14 VideosPERIODIC TABLE
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|37 VideosPRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-5 (Integer answer Type questions)|6 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-POLYMERS-Practice Sheet-1 (Linked comprehension type questions)
- Based on the behaviour of polymer it can be classified int two forms i...
Text Solution
|
- Based on the behaviour of polymer it can be classified int two forms i...
Text Solution
|
- Based on the behaviour of polymer it can be classified int two forms i...
Text Solution
|
- Compound (D) is
Text Solution
|
- Compound (E) is
Text Solution
|
- Polymer (F) is
Text Solution
|