Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
POLYMERS
AAKASH SERIES|Exercise PROBLEMS|32 VideosPOLYMERS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (SHORT ANSWER QUESTIONS)|9 VideosPOLYMERS
AAKASH SERIES|Exercise Practice Sheet -5 (Match the following questions)|2 VideosPERIODIC TABLE
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|37 VideosPRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-5 (Integer answer Type questions)|6 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-POLYMERS-Practice Sheet -5 (Integer answer Types Questions)
- How many of them are bio-degradable Ester linkage polymer PHB, PHBV,...
Text Solution
|
- How many number carbon atoms in perlon L monomer ?
Text Solution
|
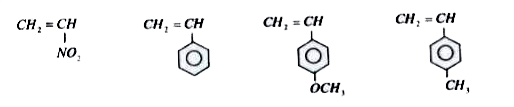
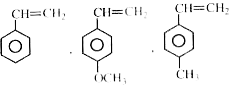
- How many of them are more reactive than CH(2)=CH(2) in the cationic po...
Text Solution
|
- How many of them are more reactive than CH(2) = CH(2) in the anionic p...
Text Solution
|
- How many of the following polymeric structures are correctly matched
Text Solution
|
- The number of polymers havng stronger intermolecular forces of attract...
Text Solution
|