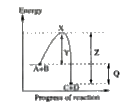
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH SERIES|Exercise LEVEL-II(LECTURE SHEET EXERCISE-II LINKED COMPREHENSION TYPE QUESTIONS)|9 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise LEVEL-II(LECTURE SHEET EXERCISE-III MATCH THE FOLLOWING QUESTIONS)|3 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise LEVEL-1 (EXERCISE -II ORDER, MOLECULARITY, HALF LIFE:)|34 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Additional Practice Exercise|54 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL KINETICS-LEVEL-II(LECTURE SHEET EXERCISE-I SINGLE & ONE OR MORE THAN ONE CORRECT ANSWER)
- For the reaction, following data is given AtoB:K(1)=10^(15) exp ((-20...
Text Solution
|
- Fraction of reactant consumed in a first order reaction after time t i...
Text Solution
|
- Mark correct statement about given graph:
Text Solution
|
- Effective collision are those in which molecules must:
Text Solution
|
- The activation energy of a reaction is zero. The rate constant of the ...
Text Solution
|
- The rate constant is given by the equation K=Ae^(-Ea//RT). Which facto...
Text Solution
|
- Which of the following statement(s) are true regarding the log K vs 1/...
Text Solution
|
- When the temperature is raise from 300 K to 400 K the rate of a reacti...
Text Solution
|
- For the decomposition of N(2)O(5)(g) it is given that 2NOI(2)O(5(g))to...
Text Solution
|
- Unit of frequency factor A in : KL+AE^(-Ea//RT) is
Text Solution
|
- Rate constant K varies with temperature by equation log K("min"^(-1))=...
Text Solution
|
- The progress of the reaction AhArrnB with time is presented in the fig...
Text Solution
|
- Select the correct statements(s)
Text Solution
|
- Which of the following are correct?
Text Solution
|
- For a first order reaction Ato products 93.75% of A initilly taken (2M...
Text Solution
|
- The rate of constant is numerically the same for 1 order, II order and...
Text Solution
|
- For a reaction AtoB+C. It was found that at the end of 10 minutes from...
Text Solution
|
- Incorrect statements in the following
Text Solution
|
- Which of the following statement(s) is are/correct
Text Solution
|
- For a chemical reaction Ato Products, the rate of disappearance of A i...
Text Solution
|