Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH SERIES|Exercise Examples|32 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise Subjective Exercise -1 (Long answer questions)|1 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise PRACTICE SHEET-5 (MATCH THE FOLLOWING QUESTIONS)|2 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Additional Practice Exercise|54 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL KINETICS-PRACTICE SHEET-5 (INTEGER ANSWER TYPE QUESTIONS)
- For an acid catalysed reaction Aoverset(H^(+))(to)B half life period i...
Text Solution
|
- For first order parallel reactionK(1) and K(2) are 4 and 2"min"^(-1) r...
Text Solution
|
- The rate of a reaction increased from 2 units to 54 units due to chang...
Text Solution
|
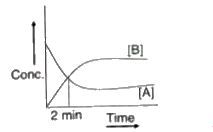
- For a first order reaction A((g))hArr3B(g) the concentration verses t...
Text Solution
|
- A chemical reaction occurs in three paths having rate constants k(1),k...
Text Solution
|
- In a closed container NO(2) gas is getting dimerised into N(2)O(4) gas...
Text Solution
|