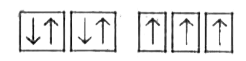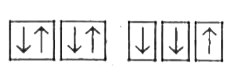A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise Objective Exericse - 2A|85 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Objective exercise - 2B|64 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Subjective Exercise - 3 (Short answer questions)|34 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMIC STRUCTURE-Objective exercise - 1
- Nitrogen atom has 3 unpaired electrons in its ground state. It can be ...
Text Solution
|
- The electronic cnfiguration of sodium is
Text Solution
|
- Which of the following represent an acetal/ ketal
Text Solution
|
- Electronic configuration of the element with atomic number 56 and mass...
Text Solution
|
- The electronic configuration of Sodium is 1s^(2) 2s^(2) 2p^(6) 3s^(1) ...
Text Solution
|
- The correct valence electronic configuration for Cu(z = 29) is
Text Solution
|
- Which one of the following pairs of ions have the same electronic conf...
Text Solution
|
- The (n+l) value for 4f sub-shell is
Text Solution
|
- The energy of the electron in the hydrogen atom is given by the expres...
Text Solution
|
- After 3d-su level is completely filled the differentiating electron en...
Text Solution
|
- The electronic configuration of chromium (Z=24) is
Text Solution
|
- The configuration 1s^(2)2s^(2) 2p^(6)3s^(2)3p^(3) corresponds to
Text Solution
|
- To which element does the following electronic configuration correspon...
Text Solution
|
- The total number of 'p' electrons present in phosphorous atom is
Text Solution
|
- Element with atomic number 38, belongs to
Text Solution
|
- Among N^(-3), O^(-2), F^(-), Na^(+), Mg^(+2) and Al^(+3) (a) What is...
Text Solution
|
- The number of unpaired electrons in the valance shell of silicon is
Text Solution
|
- a) An orbital can hold only two electrons b) An orbital must have e...
Text Solution
|
- Which of the following electronic configuration corresponds to an iner...
Text Solution
|
- The rule that explain the reason for chromium to have [Ar]3d^(5)4s^(1)...
Text Solution
|