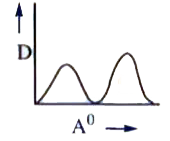
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise Exercise On Passages|19 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Level - I (EAMCET) (Exercise - I (Fundamental particle , Atomic number and mass number , Atomic models, Nature of light , Spectra))|31 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise Objective exercise - 2B|64 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMIC STRUCTURE-Objective Exercise - 3
- The velocity of electron in hydrogen atom is 7.29 xx 10^(7) cm/sec The...
Text Solution
|
- A particle of mass one microgram is confined to move along one directi...
Text Solution
|
- The set of quantum numbers 'n' and 'l' possible for the orbital shown ...
Text Solution
|
- In a multi-electron atom, which of the following orbitals described by...
Text Solution
|
- An electron has magnetic quantum number as '-3'. Its principal quantum...
Text Solution
|
- The correct match is
Text Solution
|
- Which one of the following statements is correct ?
Text Solution
|
- Which orbitals gives an electron the greatest probability of closer to...
Text Solution
|
- A radiation of wave length 3000 Å is required to remove an electron fr...
Text Solution
|
- When a certain metal was irradiated with light of frequency 3.2 xx 10^...
Text Solution
|
- If a metal is irradiated with light of frequency 3 xx 10^(19) sec^(-1)...
Text Solution
|
- True statements among the following are (A) Number of wave generated...
Text Solution
|
- The wavelength of the electron in the first orbit of the Hydrogen atom...
Text Solution
|
- If the radius of first Bohr orbit of H atom is x, then de Broglie wave...
Text Solution
|
- Uncertainity in the position of an electron (mass = 9.1 xx 10^(-31) kg...
Text Solution
|
- Assertion (A) : The orbital angular momentum of 2s-electron is equal t...
Text Solution
|
- If the Nitrogen atom had electronic configuration 1s^(7), it would hav...
Text Solution
|
- match the following .
Text Solution
|
- The expression for the Bohr's radius of hydrogen like species is
Text Solution
|
- The potential energy of the electron present in the ground state of Li...
Text Solution
|