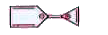Text Solution
Verified by Experts
Topper's Solved these Questions
AROMATIC HYDROCARBONS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE (Long answer questions)|6 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE (Short answer questions)|10 VideosALKALINE EARTH METALS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ADDITIONAL QUESTIONS|15 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-AROMATIC HYDROCARBONS-OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)
- Explain whether the compound shown below is aromatic or not?
Text Solution
|
- Using anhydrous AICI3 as catalyst which one of the following reactions...
Text Solution
|
- Benzene reacts with CH3Cl in the presence of anhydrous AlCl3 to form
Text Solution
|
- In the following reaction, C6H5 CH2Br underset(2.H3O^(+))overset(1."...
Text Solution
|
- Among the following compounds the one that is most reactive towards el...
Text Solution
|
- Some meta directing substituents in aromatic substitution are given wh...
Text Solution
|
- Which of the following compounds will not undergo Friedel - Crafts rea...
Text Solution
|
- Given : The enthalpy of the hydrogenation of these compounds will...
Text Solution
|
- The oxidation of benzene by V2O5 in the presence of air produces
Text Solution
|
- Consider the nitration of benzene using mixed conc. H2SO4 and HNO3 If ...
Text Solution
|
- In the given reaction the product is
Text Solution
|