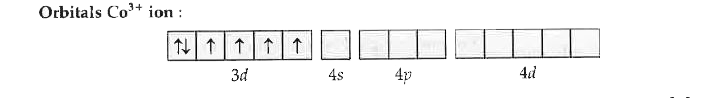
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-SOLVED PAPER (II PUC 2020)-PART-C
- Write the equations for the reactions involved in the leaching of alum...
Text Solution
|
- Write any three anamolous properties of nitrogen.
Text Solution
|
- In the manufacture of sulphuric acid write : (i) The equation with c...
Text Solution
|
- (a) Complete the following reaction : (i) NH(3) + 3Cl(2) rarr overse...
Text Solution
|
- Transition elements show catalytic property. Give two reasons.
Text Solution
|
- Write the balanced equations in the manufacture of potassium dichromat...
Text Solution
|
- Using valence bond theory explain geometry, hybridisation and magnetic...
Text Solution
|
- Indicate the type of Isomerism in the following set of complex compoun...
Text Solution
|
- Write the equations for the reactions involved in the leaching of alum...
Text Solution
|
- Mention any two anomalous properties of nitrogen.
Text Solution
|
- In the manufacture of sulphuric acid write : (i) The equation with c...
Text Solution
|
- (a) Complete the following reaction : (i) NH(3) + 3Cl(2) rarr overse...
Text Solution
|
- Transition elements show catalytic property. Give two reasons.
Text Solution
|
- Explain the manufacture of Potassium dichromate from chromite ore.
Text Solution
|
- Using valence bond theory explain geometry, hybridisation and magnetic...
Text Solution
|
- Write any two postulates of Werner's theory of co-ordination compounds...
Text Solution
|