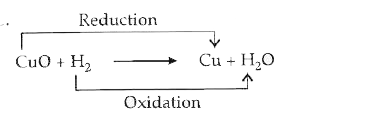Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (SHORT ANSWER TYPE QUESTION)|14 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (SHORT ANSWER TYPE QUESTION-II)|21 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (Match the Column)|1 VideosCARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise NCERT Corner (Textbook Exercises)|15 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|16 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CHEMICAL REACTIONS AND EQUATIONS-TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (Very Short Answer Type Questions)
- Why is photosynthesis considered an endothermic reaction ?
Text Solution
|
- State the type of chemical reaction used for the extraction of metals ...
Text Solution
|
- Why is hydrogen proxide kept in coloured bottles ?
Text Solution
|
- N(2)+3H(2)rarr2NH(3), name the type of reaction .
Text Solution
|
- Why do silver articles become black after sometime when exposed to air...
Text Solution
|
- Give reason: why do chips manufactures usually flush bags of chips wi...
Text Solution
|
- Identify the substances that are oxidized and the substances that are ...
Text Solution
|
- Write a balanced chemical equation for a chemical combination reactio...
Text Solution
|
- Give an example of a double - displacement reaction.
Text Solution
|
- Identify the reducing agent in the following reaction : Fe(2)O(3)+3C...
Text Solution
|