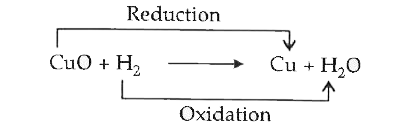
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (SHORT ANSWER TYPE QUESTION-II)|21 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (LONG ANSWER TYPE QUESTIONS)|7 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (Very Short Answer Type Questions)|10 VideosCARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise NCERT Corner (Textbook Exercises)|15 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|16 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CHEMICAL REACTIONS AND EQUATIONS-TOPIC-2 (Types of Chemical Reactions-Corrosion and Rancidity) (SHORT ANSWER TYPE QUESTION)
- What is a combination reaction ? State one example giving balanced ch...
Text Solution
|
- (i) 2PbO+Crarr2Pb+CO(2) (ii) MnO(2)+4HClrarrMnCl(2)+2H(2)O+Cl(2) W...
Text Solution
|
- Identify the type of reaction from the following equation and define i...
Text Solution
|
- Why does the colour of copper sulphate solution change when an iron ...
Text Solution
|
- (i) List any two changes which take place when oily food gets oxidize...
Text Solution
|
- A student prepares aqueous solutions of the following salts: Copper ...
Text Solution
|
- Mention the colour of FeSO(4). 7H(2)O crystals . How does this colour ...
Text Solution
|
- Write balanced equation for the rfeaction between magnesium and hydroc...
Text Solution
|
- Identify the type of reaction from the following equations: (i) CH(...
Text Solution
|
- Barium chloride reacts with aluminium sulphate to give aluminium chlor...
Text Solution
|
- When hydrogen gas is passed over heated copper (II) oxide, copper and ...
Text Solution
|
- Write the balanced chemical equation for the following reaction and id...
Text Solution
|
- Identify the oxidising agents (oxidants ) in the following reactions: ...
Text Solution
|
- A silver article generally turns black when kept in the open for a few...
Text Solution
|