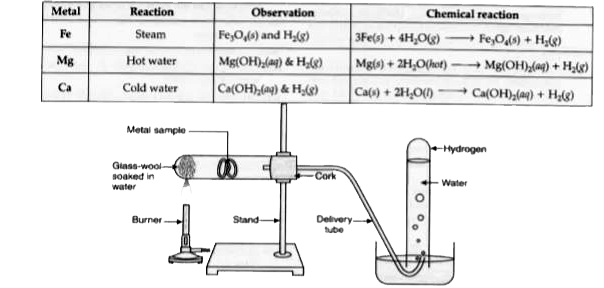
Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (MULTIPLE CHOICE QUESTIONS)|2 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise TOPIC - 2 Very Short Answer Type Questions|7 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise TOPIC - 1 Short Answer Type Questions - I|16 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|20 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise NCERT CORNER - TEXTBOOK EXERCISES|10 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-METALS AND NON-METALS-TOPIC - 1 Short Answer Type Questions - II
- List three properties of sodium by which it differs from the general p...
Text Solution
|
- Compare the properties of a typical metal and a non-metal on the basis...
Text Solution
|
- Name a non-metal which is lustrous and a metal which is liquid at the ...
Text Solution
|
- Write one example of each of the following : (i) Most malleable meta...
Text Solution
|
- (i) A non-metal X exists in two different forms Y and Z. Y is the hard...
Text Solution
|
- State reason for the following : (i) Non-metals cannot displace hydr...
Text Solution
|
- Give reason for the following : (i) Metals conduct electricity. (i...
Text Solution
|
- Give reason for the following : (i) Aluminium is a reactive metal bu...
Text Solution
|
- Arrange the following metals in the order their decreasing reactivity ...
Text Solution
|
- Give chemical equation for the reaction of aluminium powder with magan...
Text Solution
|
- Write balanced equations for the reactions of : (i) Aluminium when h...
Text Solution
|
- Write the balanced chemical equations for the following reactions : ...
Text Solution
|
- Describe an activity to show that the rusting of iron occurs in the pr...
Text Solution
|
- You are given samples of three metals : Sodium, magnesium and copper. ...
Text Solution
|
- There are 115 elements known till today. Some of them are metals and s...
Text Solution
|
- Compose an activity to arrange Ca, Mg and Fe metals in the decreasing ...
Text Solution
|
- How is the method of extraction of metals high up in the reactivity se...
Text Solution
|