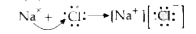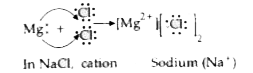Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
OSWAAL PUBLICATION|Exercise TOPIC - 2 Long Answer Type Questions|18 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Intext Questions)|15 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise TOPIC - 2 Short Answer Type Questions - I|17 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|20 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise NCERT CORNER - TEXTBOOK EXERCISES|10 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-METALS AND NON-METALS-TOPIC - 2 Short Answer Type Questions - II
- Describe electrolytic refining of copper with chemical equations. Draw...
Text Solution
|
- Define (i) mineral (ii) ore. Aluminium occurs in combined state wher...
Text Solution
|
- (i) What is meant by corrosion ? (ii) Why do aluminium sheets not co...
Text Solution
|
- Define alloys. List the properties of alloys that make them useful ove...
Text Solution
|
- (i) "Sodium is a highly reactive metal and it cannot be obtained from ...
Text Solution
|
- (i) Explain the formation of ionic compound CaO with electron dot stru...
Text Solution
|
- Give reasons for the following : (i) Ionic compounds have high melti...
Text Solution
|
- Illustrate the formation of bond in : (i) Sodium chloride (ii) Magne...
Text Solution
|
- What is cinnbar ? How is a metal extracted from cinnabar ? Explain bri...
Text Solution
|
- Zinc is a metal found in the middle of the activity series of metals. ...
Text Solution
|
- Which method will you use to reduce the following ? (i) Oxides of le...
Text Solution
|
- In a thermite reaction, a compound of iron reacts with a metal : (i)...
Text Solution
|
- Zinc is the metal which lies in the middle of the activity series. Thi...
Text Solution
|