Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|16 VideosMETALS AND NON-METALS
OSWAAL PUBLICATION|Exercise TOPIC - 2 Long Answer Type Questions|18 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|20 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise NCERT CORNER - TEXTBOOK EXERCISES|10 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-METALS AND NON-METALS-NCERT CORNER (Intext Questions)
- Give an example of a metal which (i) is a liquid at room temperature...
Text Solution
|
- Explain the meanings of malleable and ductile.
Text Solution
|
- Why is sodium kept immersed in kerosene oil ?
Text Solution
|
- Write equations for the reactions. (i) Iron with steam (ii) Calciu...
Text Solution
|
- Samples of four metals A, B, C and D were taken and added to the follo...
Text Solution
|
- Which gas in produced when dilute hydrochloric acid as added to reacti...
Text Solution
|
- What would you observe when zinc is added to a solution of iron (II) s...
Text Solution
|
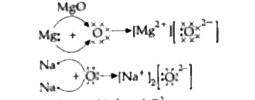
- (i) Write the electron dot structure for sodium, oxygen and magnesium....
Text Solution
|
- Why do ionic compounds have high melting point ?
Text Solution
|
- Define the following terms. (i) Mineral (ii) ore (iii) Gangue
Text Solution
|
- Name two metals which are found in nature in a free state.
Text Solution
|
- What chemical process is used for obtaining a metal from its oxide ?
Text Solution
|
- Metallic Oxides of zinc, magnesium and copper were heated with the fol...
Text Solution
|
- Which metals do not corrode easily ?
Text Solution
|
- What are alloys ?
Text Solution
|