Similar Questions
Explore conceptually related problems
Recommended Questions
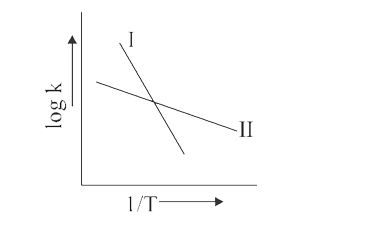
- The Arrhenius relationship of two different reaction is shown below: ...
Text Solution
|
- An endothermic reaction proceeds faster in the forward reaction with d...
Text Solution
|
- Assertion (A) : If the activation energy of a reaction is zero, temper...
Text Solution
|
- The Arrhenius relationship of two different reactions is shown below. ...
Text Solution
|
- Which of the two reactions proceed faster ?
Text Solution
|
- The Arrhenius relationship of two different reactions is shown below. ...
Text Solution
|
- The Arrhenius plots of two reactions, I and II are shown graphically- ...
Text Solution
|
- Assertion : If the activation energy of a reaction is zero , temperatu...
Text Solution
|
- उच्च सक्रियण ऊर्जा वाली अभिक्रिया कम सक्रियण ऊर्जा वाली अभिक्रिया की अ...
Text Solution
|