Similar Questions
Explore conceptually related problems
Recommended Questions
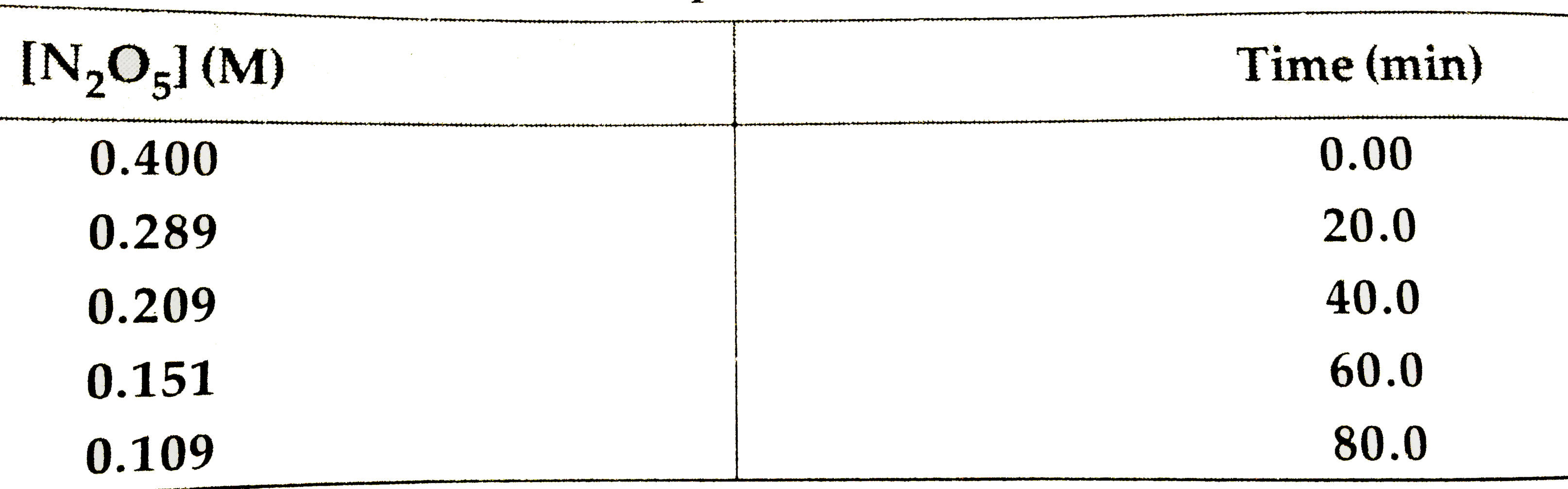
- Nitrogen pentoxide decomposes according to equation : 2N2O5(g)to4NO...
Text Solution
|
- Nitrogen pentoxide decomposes according to equation 2N(2)O(5)(g) to ...
Text Solution
|
- Nitrogen pentoxide decomposes according to equation : 2N2O5(g)to4NO...
Text Solution
|
- 2N2O5(g)rarr4NO2(g)+O2(g) what is the ratio of the rate of decompositi...
Text Solution
|
- Nitrogen pentoxide decomposes according to equation : 2N(2)O(5)(g) r...
Text Solution
|
- In the process 2N2O5(g) to 4NO2(g) + O2(g) , at t = 10 rate of reactio...
Text Solution
|
- Nitrogen pentoxide decomposes according to equation : 2N2O5(g)to4NO...
Text Solution
|
- For the first order reaction. N2O5(g) rightarrow N2O4(g) + 1/2 O2 (g),...
Text Solution
|
- For the reaction 2N2O5(g) rarr 4NO2(g)+O2(g) the following results hav...
Text Solution
|